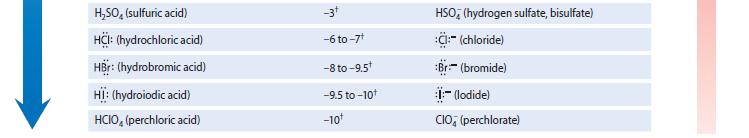
Using the pK a values in Table 3.1, calculate the equilibrium constant for each of the following
Question:
Using the pKa values in Table 3.1, calculate the equilibrium constant for each of the following reactions.
(a) NH3 acting as a base toward the acid HCN
(b) F– acting as a base toward the acid HCN
Transcribed Image Text:
GREATER ACIDITY TABLE 3.1 Relative Strengths of Some Acids and Bases Conjugate acid pk NH, (ammonia) RÖH (alcohol) HÖH (water) HPO (hydrogen phosphate) RSH (thiol) R₂NH (trialkylammonium ion) NH, (ammonium ion) HCN (hydrocyanic acid) H₂PO, (dihydrogen phosphate) HSH (hydrosulfuric acid) R-C-OH (carboxylic acid) HF: (hydrofluoric acid) H₂PO4 (phosphoric acid) HNO, (nitric acid) H₂O* (hydronium ion) H₂C- -SO₂H (p-toluene- sulfonic acid) ~35t 15-19 15.7 12.3 10-12* 9-11* 9.25 9.40 7.21 7.0 4-5* 3.2 2.2 -1.3 -1.7 -2.8+ Conjugate base -NH₂ (amide) RÖ:- (alkoxide) HÖ:- (hydroxide) PO (phosphate) RS (thiolate) R₂N: (trialkylamine) H₂N: (ammonia) -:CN (cyanide) HPO (hydrogen phosphate) HS:- (hydrosulfide) R-C-Ö: (carboxylate) F:-(fluoride) H₂PO (dihydrogen phosphate) NO; (nitrate) H₂O (water) H₂C- (p-toluene- -SO sulfonate, or "tosylate") GREATER BASI CITY
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
a b The acidbase reaction is HCN NH3 CN NH4 When NH3 ...View the full answer

Answered By

Shameen Tahir
The following are details of my Areas of Effectiveness. The following are details of my Areas of Effectiveness English Language Proficiency, Organization Behavior , consumer Behavior and Marketing, Communication, Applied Statistics, Research Methods , Cognitive & Affective Processes, Cognitive & Affective Processes, Data Analysis in Research, Human Resources Management ,Research Project,
Social Psychology, Personality Psychology, Introduction to Applied Areas of Psychology,
Behavioral Neurosdence , Historical and Contemporary Issues in Psychology, Measurement in Psychology, experimental Psychology,
Business Ethics Business Ethics An introduction to business studies Organization & Management Legal Environment of Business Information Systems in Organizations Operations Management Global Business Policies Industrial Organization Business Strategy Information Management and Technology Company Structure and Organizational Management Accounting & Auditing Financial Accounting Managerial Accounting Accounting for strategy implementation Financial accounting Introduction to bookkeeping and accounting Marketing Marketing Management Professional Development Strategies Business Communications Business planning Commerce & Technology Human resource management General Management Conflict management Leadership Organizational Leadership Supply Chain Management Law Corporate Strategy Creative Writing Analytical Reading & Writing Other Expertise Risk Management Entrepreneurship Management science Organizational behavior Project management Financial Analysis, Research & Companies Valuation And any kind of Excel Queries.
4.70+
16+ Reviews
34+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Find the total capacitance CT of the network in figure. 3 F CT 4 uF 6 F 6 F 3 F
-
Using the pka values in Table 3.1, calculate the equilibrium constant for the following reaction. F- acting as a base toward the acid HCN
-
Using the pKa values in Table 17.1, predict the products of the following reactions: a. b. c. d. OCH3 CH3 NaOH CH3 Table 17.1 The pKa Values of the Conjugate Adds of the Leaving Groups of Carbonyl...
-
Complete the following table of basic calculations. For Percent Contribution Margin, use (P-MC)/P. Round to table standard. Price 18 17 16 15 14 13 j Quantity Demanded 600 a 800 900 1000 1100 1200...
-
Did the defendants conduct constitute ordinary negligence, meaning they are not liable under 519(b), or gross negligence, meaning they are liable?
-
Based on their respective van der Waals constants (Table 10.3), is Ar or CO2 expected to behave more nearly like an ideal gas at high pressures? Explain. TABLE 10.3Van der Waals Constants for Gas...
-
3. Using the methodology outlined in Exhibit 11.11, forecast the operating items on the next five years of balance sheets for PartsCo. Forecast each balance sheet item as a function of revenues,...
-
The inventory records of Kuffel Co. reflected the following information for the year ended December 31, 2010: Required: a. Assume that Kuffel Co. uses a periodic inventory system. Calculate cost of...
-
AutoPartsWarehouse.com purchases chrome rims for $740 (for a set of 4) and uses a markup of 25% on selling price. What will be the percent markup on cost? Report your answer as a a percentage and...
-
Write an equation for each of the following equilibria, and use Table 3.1 to identify the pK a value associated with the acidic species in each equilibrium. (a) Ammonia acting as a base toward the...
-
What is the dissociation constant of an acid that has each of the following pK a values? (a) 4 (b) 7.8 (c) 2
-
Under Par, Inc., is an Internet retailer of golf equipment. Customers order golf equipment from the company, using an online catalog. The company processes these orders and delivers the requested...
-
State the vertical asymptotes, if any exist for the function. T f(x) = x+81
-
4. Oh no! Prof. Conlin was doing the dishes, but ran out of space on his drying rack. He decided to set the last two bowls on a towel on the counter to dry. He wondered, "To make sure they get dry,...
-
It has been assumed so far that the firm will operate a project over its full physical life. However, may not be the best option - it may be better to abandon a project prior to the end of potential...
-
33-34 Find (a) f + g, (b) f- g, (c) fg, and (d) f/g and state their domains. 33. f(x)=25-x, g(x) = x+1 ===== 1 34. f(x)= x-1' 9(x)=-2 X
-
Describe five steps independent auditors take when auditing an organization. -An independent auditor, often a public accounting firm, begins an audit by studying the business. This approach helps to...
-
List at least three functions of proteins.
-
CLASS PERIO Solving Linear Equations: Variable on Both Sides Solve each equation. 1) 6r+ 7 = 13 + 7r 3) -7x-3x+2=-8x-8 5)-14 +66+7-26=1+5b 7) n-3n = 14-4n 2) 13-4x=1-x 4)-8-x= x - 4x 6)n+2=-14-n 8)...
-
When a mixture of methane and chlorine is irradiated, reaction commences immediately. When irradiation is stopped, the reaction gradually slows down but does not stop immediately. Explain.
-
Radical chlorination of pentane is a poor way to prepare 1-chioropentane, but radical chlorination of neopentane, (CH3)4C, is a good way to prepare neopentyl chloride, (CH3)3CCH2C1. Explain.
-
Despite the limitations of radical chlorination of alkanes, the reaction is still useful for synthesizing certain halogenated compounds. For which of the following compounds does radical chlorination...
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...

Study smarter with the SolutionInn App


