A compound with molecular formula C 7 H 14 O exhibits the following 13 C NMR spectra:
Question:
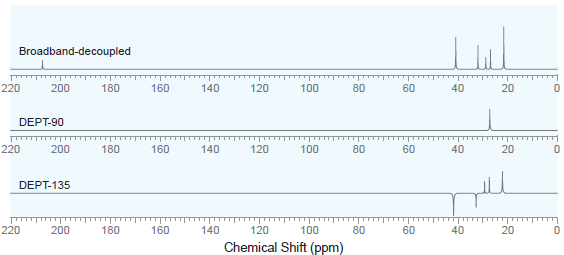
Several structures are consistent with these spectra. To determine which structure is correct, a 1H NMR spectrum was acquired which exhibits six signals. One of those signals is a singlet at 1.9 ppm with an integration of 3, and another of the signals is a doublet at 0.9 ppm with an integration of 6. Using this information, identify the correct structure of the compound.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





