Treatment of an ?, ? unsaturated ketone with basic aqueous hydrogen peroxide yields an epoxy ketone. The
Question:
Treatment of an ?, ? unsaturated ketone with basic aqueous hydrogen peroxide yields an epoxy ketone. The reaction is specific to unsaturated ketones; isolated alkene double bonds do not react. Propose a mechanism.
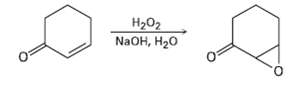
На02 NaOH, H20 O.
Step by Step Answer:
HOOH OH Hydrogen peroxide and hydroxide react to form water and ...View the full answer



Related Video
Hydrogen peroxide can be used as a mild antiseptic to curb superficial skin infections such as athlete’s foot, but only in diluted quantities. To combat stinky feet, try soaking your feet in a solution of 1 part 3% hydrogen peroxide and 3 parts warm water for 15-20 minutes, then drying them thoroughly. This will kill odor-causing bacteria and soften your feet. To treat athlete\'s foot, you can use a similar solution, but only in diluted quantities, and soak your feet for 30 minutes. Hydrogen peroxide can also be used to keep vegetables fresh by adding 1/4 cup to a bowl of cold water, soaking the vegetables for 20-30 minutes, then draining, drying, and refrigerating them. Alternatively, you can spray vegetables with a solution of 3% hydrogen peroxide and let them stand for a few minutes before rinsing and drying. To keep leftover salad fresh, spray it with a solution of 1/2 cup water and 1 Tbsp. 3% hydrogen peroxide, drain, cover, and refrigerate.
Students also viewed these Organic Chemistry questions
-
Hydrogen peroxide in aqueous solution decomposes by a first-order reaction to water and oxygen. The rate constant for this decomposition is 7.40 104/s. What quantity of heat energy is initially...
-
Hydrogen peroxide and ferrous sulfate react to produce hydroxyl radical (HO), as reported in 1894 by English chemist H. J. H. Fenton. When tert-butyl alcohol is treated with HO generated this way, it...
-
Propose a mechanism for the reaction of cyclohexyl methyl ketone with excess bromine in the presence of sodium hydroxide.
-
Write a detailed executive summary about the appropriation and advancement of Information and Communication Technology (ICT)
-
Groupthink is the tendency for teams to put such a high premium on agreement that directly (or indirectly) punish dissent. Was the Schumacker's team guilty of groupthink? Why or why not?
-
What role does a mentor play in leadership development?
-
Suppose interest rates on residential mortgages of equal risk were 7 percent in California and 9 percent in New York. Could this differential persist? What forces might tend to equalize rates? Would...
-
An Ionic Crystal Figure shows eight point charges arranged at the corners of a cube with sides of length d. The values of the charges are +q and -q, as shown. This is a model of one cell of a cubic...
-
If you double-click on a CSV file in Windows Explorer, it open automatically in Excel, how else can you open a CSV file so that you can view the actual content of the file? Use the Text Import Wizard...
-
The file Banks.csv includes data on a sample of 20 banks. The Financial Condition column records the judgment of an expert on the financial condition of each bank. This outcome variable takes one of...
-
Tran?s alkenes are converted into their cis isomers and vice versa on epoxidation followed by treatment of the epoxide with triphenylphosphine. Propose a mechanism for the epoxide ? alkene reaction. ...
-
One of the biological pathways by which an amine is converted to a ketone involves two steps: (1) oxidation of the amine by NAD+ to give an imine, and (2) hydrolysis of the imine to give a ketone...
-
We?ll see that there are two isomeric substances both named 1, 2-dimethylcyclohexane. Explain. -C3 1,2-Dimethylcyclohexane CH
-
The four forces, 400, 500, 600 and 700N are acting along the edges of a 0.8m cube as shown. Represent the resultant of these forces by 1) A force Fr through the point A 2) A couple moment Mr (give...
-
Problem 1. What is the degree of freedom of the following mechanism? Sliding joint Sliding joint
-
PILAR Manufacturing Co. has three producing departments (P, I, & L), and two service departments (A&R). The total estimated departmental expenses for 2021 before distribution of service department...
-
1. A volleyball player serves the ball at point A with an initial velocity vo at an angle of 20 to the horizontal. (a) Determine the minimum velocity of the serve such that the ball will just clear...
-
9.50. Dipping low ** A top with I = 3/3 floats in outer space and initially spins around its x3 axis with angular speed w3. You apply a strike at the bottom point, directed into the page, as shown in...
-
Audit engagement programs testing internal controls should: (a) Be tailored for the audit of each operation. (b) Be generalized to fit all situations without regard to departmental lines. (c) Be...
-
The diameter of a sphere is 18 in. Find the largest volume of regular pyramid of altitude 15 in. that can be cut from the sphere if the pyramid is (a) square, (b) pentagonal, (c) hexagonal, and (d)...
-
Use data from Table 10.3 to estimate the enthalpy change ( r H) for the following reaction. Table 10.3 CH6(g) + Cl2(g) CH5Cl(g) + HCl(g) AH = ?
-
Show how you might prepare aniline from each of the following compounds: (a) Benzene (b) Bromobenzene (c) Benzamide
-
Show how you might synthesize each of the following compounds from 1-butanol: (a) Butylamine (free of 28 and 38 amines) (b) Pentylamine (c) Propylamine (d) Butylmethylamine
-
Show how you might convert aniline into each of the following compounds. (You need not repeat steps carried out in earlier parts of this problem.) (a) Acetanilide (b) N-Phenylphthalimide (c)...
-
Practicum Co. pad $1.2 million for an 80% interest in the common stock of Sarong Co. Practicum had no previous equity interest in Sarong. On the acquisition date, Sarong's identifiable net assets had...
-
On Dec 31 2020, Bernice Melson, a partner in ABC Communications, had an ending capital balance of $49,000. Her share of the partnership's profit was $18,000; she made investments of $12,000 and had...
-
Q2R. on account for each depreciable asset. During 2024, Jane VIIS nsactions.) i More Info Apr. 1 Purchased office equipment. 5111,000. Paid 581,000 cash and financed the remainder Jan. 1 with a note...

Study smarter with the SolutionInn App


