Tran?s alkenes are converted into their cis isomers and vice versa on epoxidation followed by treatment of
Question:
Tran?s alkenes are converted into their cis isomers and vice versa on epoxidation followed by treatment of the epoxide with triphenylphosphine. Propose a mechanism for the epoxide ? alkene reaction.
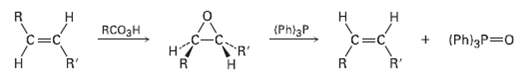
Transcribed Image Text:
н RCO3H (Phl3P R' (Ph)3P 0 на R' R'
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 38% (13 reviews)
Ph3P H Ph3P H rotate R 180 R H Ph3P rotatio...View the full answer

Answered By

Wahome Michael
I am a CPA finalist and a graduate in Bachelor of commerce. I am a full time writer with 4 years experience in academic writing (essays, Thesis, dissertation and research). I am also a full time writer which assures you of my quality, deep knowledge of your task requirement and timeliness. Assign me your task and you shall have the best.
Thanks in advance
4.90+
63+ Reviews
132+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Propose a mechanism for the following reaction (remember to use curved arrows when showing a mechanism): CH3CHCH-CH-OH CH,C-CH, CH,CHCH-CH-OCCH, + CH CH
-
Propose a mechanism for the following reaction that explains why the configuration of the asymmetric carbon in the reactant is retained in the product: COO NaNO2 HCl NH2 COO
-
Propose a mechanism for reaction of the first three propylene units in the polymerization of propylene in the presence of a peroxide. ROOR- H2C CHCH high pressure C-C propylene I7 polypropylene
-
Cubitene is a diterpene present in the defense secretion of a species of African termite. What unusual feature characterizes the joining of isoprene units in cubitene?
-
A procedural leader sets an agenda, makes sure that everyone knows what's due for the next meeting, and checks to be sure that tasks are carried out. Does Malcom fulfill the role of an effective...
-
Fill in the missing amounts in the following income statement for Carpenters Department Store Inc. $125,600 Sales revenue Less: Sales returns and allowances (a) Net sales $122,040 Cost of goods sold:...
-
What would happen to the standard of living in the United States if people lost faith in the safety of our financial institutions? Why? AppendixLO1
-
Amanda King has just been appointed director of recreation programs for Highland Park, a rapidly growing community in Connecticut. In the past, the city has sponsored a number of softball leagues in...
-
Burnett, Inc.. has current assets of $6800, net fixed assets of $29,400, current liabilities of $5,400, and long term debt of $13,100. What is the value of the shareholder's equity account for this...
-
Volcano Potato Company (VPC) grows potatoes, processes them, and then sells three potato products: fresh potatoes, frozen french fried potatoes, and frozen hash ball potatoes (shredded and then...
-
In light of ?our answer to Problem 19.56, propose a mechanism for the formation of 3, 5 -dimethylisoxazole from hydroxylamine and 2, 4-pentanedione. CH 3,5-Dimethylisoxazole
-
Treatment of an ?, ? unsaturated ketone with basic aqueous hydrogen peroxide yields an epoxy ketone. The reaction is specific to unsaturated ketones; isolated alkene double bonds do not react....
-
Troy's 2009 tax return is audited. The auditor determines that Troy inadvertently understated his ending inventory in calculating his business income. The error creates an additional tax liability of...
-
Let f(x) = x+ 3, x20. The inverse of f is Of 1(x)=x - 3 (f (x) = -x-3 f-(x) = x - 3 Of 1(x) = 3 - x
-
Read the articles and please help me to write the whole assignment perfectly including the citations and references (APA Format). Pleaase choose the country and perspective of a particular industry....
-
A light, inextensible cord passes over a frictionless pulley as shown in figure below. One end of the rope is attached to a block, and a force P is applied to the other end. Block A weighs 600 lb and...
-
BASICOT POST DO NOT ASSIST DO NOT POST DO NOT ASSIST DO NOT POST DO NOT ASSIST For filming a physics demonstration about oscillation, an educational video crew attaches a large spring to a very small...
-
Day Mail Order Co. applied the high-low method of cost estimation to customer order data for the first 4 months of the year. What is the estimated variable order-filling cost component per order...
-
Which of the following audit procedures would be most effective in determining if purchasing requirements have been updated for changes in production techniques? (a) Recalculate parts needed based on...
-
In the operation of an automated production line with storage buffers, what does it mean if a buffer is nearly always empty or nearly always full?
-
One of the chemical reactions that occurs in the formation of photochemical smog is O 3 + NO NO 2 + O 2 . Estimate r H for this reaction by using appropriate Lewis structures and data from Table...
-
Give common or systematic names for each of the following compounds: (a) (b) (c) (d) (e) (f) (g) (h) (i) (j) (k) CH3N+H3 CH3CO2 - (l) (m) (n) NH2 HIN NH2 CH3 NH2 OCH NIH N NH2 SO NH2 NH2 CH3
-
Which is the most basic nitrogen in each compound. Explain your choices. (a) (b) (c) NH2 HN
-
Show how you might prepare benzylamine from each of the following compounds: (a) (b) (c) Benzyl bromide (two ways) (d) Benzyl tosylate (e) Benzaldehyde (f) Phenylnitromethane (g) NH2 Benzylamine CN...
-
All else constant, if the yield to maturity of a bond increases, the the value of the bond __________. a. increases b. decreases c. remains the same d. not enough information To answer enter a, b, c,...
-
Martha s Vineyard Marine Supply is a wholesaler for a large variety of boating and fishing equipment. The company s controller, Mathew Knight, has recently completed a cost study of the firm s...
-
1. Compute the productivity profiles for each year. If required, round your answers to two decimal places. 2a. Did productivity improve? 2b. Explain why or why not

Study smarter with the SolutionInn App


