In light of ?our answer to Problem 19.56, propose a mechanism for the formation of 3, 5
Question:
In light of ?our answer to Problem 19.56, propose a mechanism for the formation of 3, 5 -dimethylisoxazole from hydroxylamine and 2, 4-pentanedione.
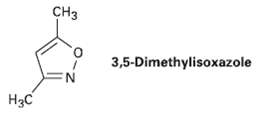
Transcribed Image Text:
CHз 3,5-Dimethylisoxazole Нас
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (15 reviews)
The same sequence of steps used in the previous problem ...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Propose a mechanism for the formation of D-fructose-1,6-diphosphate from dihydroxyacetone phosphate and D-glyceraldehyde-3-phosphate, using HO- as the catalyst.
-
Propose a mechanism for the formation of Melmac.
-
Propose a mechanism for the following reaction. excess HBr Br tetrahydrofuran 1,4-dibromobutan
-
Through the use of strategic alternatives, companies may compete in a marketplace, achieve its vision, or if no vision has been articulated, decide where it might go and what it might achieve....
-
1. Of those veterans who would like to work for Team Rubicon, what types of rewards are likely to keep them motivated: extrinsic, intrinsic, or both? 2. To what degree do you think Team Rubicon will...
-
What are the rules of debit and credit as they apply to the contra-asset account Accumulated Depreciation?
-
How does a cost-efficient capital market help to reduce the prices of goods and services? AppendixLO1
-
The Excel file Salary Data provides information on current salary, beginning salary, previous experience (in months) when hired, and total years of education for a sample of 100 employees in a firm....
-
JEN Corp. is expected to pay a dividend of $4.00 per year indefinitely. If the appropriate rate of return on this stock is 10 percent per year, and the stock consistently goes ex-dividend 10 days...
-
Brabham Enterprises manufactures tires for the Formula I motor racing circuit. For August 2013, it budgeted to manufacture and sell 3,000 tires at a variable cost of $74 per tire and total fixed...
-
Propose a mechanism to account for the formation of 3, 5-dimethylpyrazole from hydrazine and 2, 4-pcntancdionc. Look carefully to see what has happened to each carbonyl carbon in going from starting...
-
Tran?s alkenes are converted into their cis isomers and vice versa on epoxidation followed by treatment of the epoxide with triphenylphosphine. Propose a mechanism for the epoxide ? alkene reaction. ...
-
The topic of energy would address sustainability by which of the GRI standpoints? a. Economic b. Environmental c. Social d. Financial
-
7. This is a question about electromagnetic waves. (a) Starting from Maxwell's equations in a vacuum show that the electric field E and magnetic field B obey wave equations and identify the velocity...
-
Participate in workplace health and safety Third- party report Task 1: Case scenario: Workplace hazard collection and risk control form You are required to review this workplace inspection form and...
-
The red curve is the position-time x-t graph for the ladybug. Each tick mark on the time axis of the graph marks off 0.5 s. Note: you can hit reset graph and graph again to watch the graph form again...
-
Oma's Bakery is thinking about replacing the convection oven with a new, more energy-efficient model. Information related to the old and new ovens follows: (Click the icon to view the information...
-
One could argue that substantial travel for work is an undesirable characteristic of any job. What would the theory of compensating differentials predict about the relative wages of a sales position...
-
The production line has experienced shut-downs because needed production parts were not on hand. Which of the following audit procedures would best identify the cause of the parts shortages? (a)...
-
What is a manufacturing system?
-
Estimate the standard enthalpies of formation at 25 C and 1 bar of (a) OH(g); (b) N 2 H 4 (g). Write Lewis structures and use data from Table 10.3, as necessary. Table 10.3 TABLE 10.3 Some Average...
-
(a) Starting with aniline and assuming that you have 2-aminothiazole available, show how you would synthesize sulfathiazole. (b) How would you convert sulfathiazole to succinylsulfathiazole?...
-
Write structural formulas for each of the following compounds: (a) Benzylmethylamine (b) Triisopropylamine (c) N-Ethyl-N-methylaniline (d) m-Toluidine (e) 2-Methylpyrrole (f) N-Ethylpiperidine (g)...
-
Outline a procedure for separating a mixture of benzoic acid, 4-methylphenol, aniline,and benzene using acids, bases, and organic solvents.
-
Suppose you bought a bon with an annual coupon rate of 6.5 percent one year ago for $1,032. The bond sells for $1,020 today. a. Assuming a $1,000 face value, what was your total dollar return on this...
-
During the year 2021, William has a job as an accountant, he earns a salary of $100,000. He has done some cleaning services work on his own (self-employed), where he earned a net income of $50,000....
-
Fixed cost per unit is $7 when 25,000 units are produced and $5 when 35,000 units are produced. What is the total fixed cost when 30,000 units are produced? Group of answer choices $150,000....

Study smarter with the SolutionInn App


