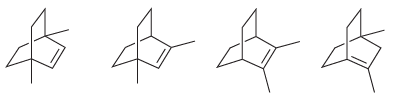
Arrange the following alkenes in order of increasing stability:
Question:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (17 reviews)
this comound is ...View the full answer

Answered By

Jinah Patricia Padilla
Had an experience as an external auditor in Ernst & Young Philippines and currently a Corporate Accountant in a consultancy company providing manpower to a 5-star hotel in Makati, Philippines, Makati Diamond Residences
5.00+
120+ Reviews
150+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Arrange the following alkenes in order of decreasing stability: 1-pentene; (E)-2-pentene; (Z)-2-pentene; 2-methyl-2-butene.
-
Arrange the following alkenes in order of decreasing stability: 1-pentene; (E)-2-pentene; (Z)-2-pentene; 2-methyl-2-butene.
-
Arrange these alkenes in order of increasing rate of reactio0n with HCI: CH, CH2=CH2 a) CH;CH2CH=CH2 CH;CH,C=CH, -CH=CH; CH=CH2 CH,CH-CH b) CHO
-
Jogger 1 is travelling east at 6 . 5 m / s and has a mass of 8 2 kg . Jogger 2 is travelling north at 5 . 8 m / s and has a mass of 5 4 . 5 kg . One of the joggers has their head down and doesnt see...
-
For this assignment you will be required view the following two videos on YouTube. www.youtube.com/watch?v=bKJPxCPB-RE www.youtube.com/watch?v=rUns1Jbve0w 1) After watching the video please indicate...
-
As a fighter jet was coming in for a landing, it was 325 ft above ground when it was directly above the end of the landing strip. If it then came in at a constant angle of 6.5 with the landing strip,...
-
In an income statement based on USHA, what information is reported in the operated departments section?
-
Mary Karston was hired by a popular fast-food restaurant as an order-taker and cashier. Shortly after taking the job, she was shocked to overhear an employee bragging to a friend about shortchanging...
-
11 Parool Problem 4.L04.13 You bastowed 300.000 Isted over 25 years. How years achi Thesis was award Thess llave you love the first year a ry date of 082 The Mond to the nearest dolar) parts showing...
-
Determine the propagation delay and contamination delay of the circuit in Figure 2.84. Use the gate delays given in Table 2.8. Table 2.8 Gate delays for Exercises 2.432.47 (sd) Pdq 15 Gate ted (ps)...
-
Angela owes $500 on a credit card and $2,000 on a student loan. The credit card has a 15 percent annual interest rate and the student loan has a 7 percent annual interest rate. Her sense of loss...
-
For each pair of the following compounds identify which compound would react more rapidly in an E1 reaction. a. b. CI .CI CI CI
-
Explain how a company can generate cash by: A. Operations B. Investing activities C. Borrowing D. Issuing shares
-
Administrators at International University are curious how students' GPAs after their first year compare to their high school GPAs. They plan on taking an SRS of 80 of the 900 freshmen to look up...
-
( 8 x - x ^ 2 ) / ( x ^ 4 ) what is the derivate.
-
Solve for x . log 1 0 ( 4 x ) log 1 0 ( x 3 ) = 1
-
Let f ( x ) = ( 8 x - 4 x ^ 2 ) It is ^ x . Find the inflection points
-
f ( x ) = sin ( x ) / ( 2 * x ^ 2 + 4 ) , differentiate using quotient with respect to x
-
Factor by grouping. 2k + 2h + jk + jh
-
Modify the counter from Exercise 5.44 such that the counter will either increment by 4 or load a new 32-bit value, D, on each clock edge, depending on a control signal Load. When Load = 1, the...
-
Intermolecular aldol cyclization of 2, 5-heptanedione with aqueous NaOH yields a mixture of two enone products in the approximate ratio 9:1. Write their structures, and show how each is formed.
-
The major product formed by intermolecular aldol cyclization of 2,5-heptanedione has two singlet absorptions in the 1 H NMR spectrum at 16.5 and 1.90 , and has no absorption in the range 3 to 10 ....
-
Treatment of the minor product formed in the intermolecular aldol cyclization of 2, 5-heptanedione with aqueous NaOH convert it into the major product. Propose a mechanism to account for this...
-
Berbice Inc. has a new project, and you were recruitment to perform their sensitivity analysis based on the estimates of done by their engineering department (there are no taxes): Pessimistic Most...
-
#3) Seven years ago, Crane Corporation issued 20-year bonds that had a $1,000 face value, paid interest annually, and had a coupon rate of 8 percent. If the market rate of interest is 4.0 percent...
-
I have a portfolio of two stocks. The weights are 60% and 40% respectively, the volatilities are both 20%, while the correlation of returns is 100%. The volatility of my portfolio is A. 4% B. 14.4%...

Study smarter with the SolutionInn App


