As described in the Practically Speaking box in Section 27.7, poly(vinyl alcohol) is used as a precursor
Question:
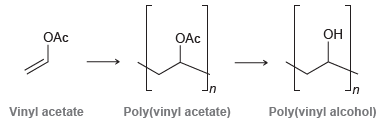
This two-step process is required because poly(vinyl alcohol) cannot be made from its corresponding monomer. Explain why vinyl alcohol will not directly undergo polymerization.
Transcribed Image Text:
OAc Он OAc in Poly(vinyl acetate) Poly(vinyl alcohol) Vinyl acetate
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (10 reviews)
Vinyl alcohol is an enol which is no...View the full answer

Answered By

Ali Khawaja
my expertise are as follows: financial accounting : - journal entries - financial statements including balance sheet, profit & loss account, cash flow statement & statement of changes in equity -consolidated statement of financial position. -ratio analysis -depreciation methods -accounting concepts -understanding and application of all international financial reporting standards (ifrs) -international accounting standards (ias) -etc business analysis : -business strategy -strategic choices -business processes -e-business -e-marketing -project management -finance -hrm financial management : -project appraisal -capital budgeting -net present value (npv) -internal rate of return (irr) -net present value(npv) -payback period -strategic position -strategic choices -information technology -project management -finance -human resource management auditing: -internal audit -external audit -substantive procedures -analytic procedures -designing and assessment of internal controls -developing the flow charts & data flow diagrams -audit reports -engagement letter -materiality economics: -micro -macro -game theory -econometric -mathematical application in economics -empirical macroeconomics -international trade -international political economy -monetary theory and policy -public economics ,business law, and all regarding commerce
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The energy-level diagram in Figure 9.36 shows that the sideways overlap of a pair of p orbitals produces two molecular orbitals, one bonding and one anti-bonding. In ethylene there is a pair of...
-
The "Chemistry and Life" box in Section 6.7 described the techniques called NMR and MRI. (a) Instruments for obtaining MRI data are typically labeled with a frequency, such as 600 MHz. Why do you...
-
Poly (vinyl alcohol) is a useful water-soluble polymer. It cannot be prepared directly from vinyl alcohol, because of the rapidity with which vinyl alcohol (CH2=CHOH) isomerizes to acetaldehyde....
-
Fill in the Blanks The giant tubeworms found in the hydrothermal vents are called lack both a gut and a mouth. Instead, they have an organ called a (types of elements, e.g. potassium, sulfur,...
-
1. One thousand dollars is deposited into a savings account at 2.7% interest compounded annually. How many years are required for the balance to reach $1946.53? After how many years will the balance...
-
Distinguish between a mixed factorial design and a P E design. How can a design be both a mixed design and a P E design?
-
6. Verify that e r(Tt)N(d2) satisfies the Black-Scholes equation.
-
Arthur Andersen LLP was the auditor for Enron, WorldCom, Waste Management and other companies that committed fraud. Andersen was forced to shut its doors forever after a U.S. Department of Justice...
-
Security A has an expected return of 7%, a standard deviation of returns of 3.5%, a correlation coefficient with the market of -0.3%, and a beta coeficient of 0.5. Security B has an expected return...
-
A variable dielectric capacitive displacement sensor consists of two square metal plates of side 5 cm, separated by a gap of 1 mm. A sheet of dielectric material 1 mm thick and of the same area as...
-
Draw the polymer that is expected when the following monomers react under acidic conditions. NH2 [] -, H,N
-
Using vinyl acetate as your only source of carbon atoms, design a synthesis for the following polymer: OAc Vinyl acetate
-
Propose a mechanism for the following reaction. excess HBr Br tetrahydrofuran 1,4-dibromobutan
-
How has face book influenced political candidate's electoral success? What is the relationship between social media technology called face book and electoral success?
-
1. Discuss the international strategies that organizations can pursue 2. Identify and compare the various modes of of foreign market entry 3. Analyse the industry market 4. Evaluate relevant macro...
-
A bulk carrier was underway. The vessel was in ballast and hold washing was scheduled in preparation for taking the next cargo. An officer, bosun, and another deck crew conducted a risk assessment...
-
8. Neutrino radiation was observed over a certain period and the number of hours in which 0, 1, 2,... signals were received was recorded. 0 1 Number of Number of Hours with Signals per Hour This...
-
What are some advantages and disadvantages of centralization and decentralization. References: Altamimi, H., Liu, Q., & Jimenez, B. (2023). Not Too Much, Not Too Little: Centralization,...
-
Exercises 712 relate to a canonical linear programming problem with an m x n coefficient matrix A in the constraint inequality Ax b. Mark each statement True or False (T/F). Justify each answer. The...
-
What is the shape of the exponential distribution?
-
When ethyl 4-hydroxybutyrate is heated in the presence of a trace of a basic catalyst (sodium acetate), one of the products is a lactone. Propose a mechanism for formation of this lactone.
-
Complete the mechanism for this acid-catalyzed transesterification by drawing out all the individual steps. Draw the important resonance contributors for each resonance-stabilized intermediate.
-
Propose a mechanism for the following ring-opening transesterification. Use the mechanism in Problem 21-13 as a model. H+ CH3OH HO OCH,
-
you are analyzing the cost of debt for a firm. Do you know that the firms 14 year maturity, 7.8 Percent coupon bonds are selling at a price of $834. The Barnes pay interest semi annually. If these...
-
***Please answer the following using excel and showcasing the formulas/calculations used*** thank you so much Financial information on AAA Ltd. is shown below. AAA Ltd. Income Statement For the Year...
-
2. In an account Anh Paglinawan currently has $216,670.00. At a rate of 8.00% how long will it take for them to have $298,390.00 assuming semi-annually compounding? (Hint: compute the exact years, do...

Study smarter with the SolutionInn App


