Using vinyl acetate as your only source of carbon atoms, design a synthesis for the following polymer:
Question:
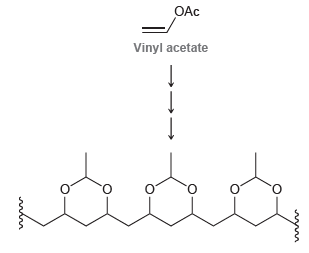
Transcribed Image Text:
OAc Vinyl acetate
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 78% (14 reviews)
OAc BF3 HO Cationic ...View the full answer

Answered By

Muhammad Haroon
More than 3 years experience in teaching undergraduate and graduate level courses which includes Object Oriented Programming, Data Structures, Algorithms, Database Systems, Theory of Automata, Theory of Computation, Database Administration, Web Technologies etc.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Using acetylene as your only source of carbon atoms, design a synthesis of pen-tanal. (Pentanal has an odd number of carbon atoms, while acetylene has an even number of carbon atoms): H.
-
Using acetylene as your only source of carbon atoms, identify a synthetic route for the production of 2-bromobutane.
-
Using acetylene as your only source of carbon atoms, identify a synthetic route for the production of 1-bromobutane.
-
Fill in the blanks in the chart below: Year Years since 1960 1960 1965 1970 1975 1980 1985 1990 1995 1996 1997 1998 1999 2000 2001 2003 2005 2006 2007 2008 2009 2010 2011 0 5 [a] [b] [c] [d] [e] [f]...
-
Give the values of i, n, P, and F. 1. $500 invested at 2.8% interest compounded annually grows to $558.40 in 4 years. 2. $800 invested on January 1, 2011, at 1.8% interest compounded monthly, grows...
-
A maze learning study with a 2 (type of maze: alley maze or elevated maze) 2 (type of rat: wild or bred in the lab) factorial has these results (DV = number of trials until performance is perfect):...
-
7. Use the answers to the previous two problems to verify that the Black-Scholes formula satisfies the Black-Scholes equation. Verify that the boundary condition V [S(T ), T ]= max[0, S(T ) K] is...
-
IHOP Corporation franchises breakfast-oriented restaurants throughout North America. The average development costs for a new restaurant were reported by IHOP as follows: Land ............. $ 667,000...
-
Philadelphia Fastener Corporation manufactures nails, screws, bolts, and other fasteners. Management is considering a proposal to acquire new material-handling equipment. The new equipment has the...
-
Edward Jones started the Jones Company Limited (JCL) as a sole proprietorship. It was later incor- porated; ownership is now 50 percent controlled by Mr. Jones, who is 71 years old, and 25 percent...
-
As described in the Practically Speaking box in Section 27.7, poly(vinyl alcohol) is used as a precursor to make PVB for use in automobile windshields. Poly(vinyl alcohol) can be prepared by...
-
When a solution of aqueous sodium hydroxide is spilled on polyester clothing, a hole develops in the fabric. Describe how the polyester is destroyed.
-
A company has a bond issue outstanding that pays $150 annual interest plus $1000 at maturity. The bond has a maturity of 10 years. Compute the value of the bond when the interest rate is 5%, 9%, and...
-
Over the past 40 years, union membership has declined, and it continues to do so. Instead, many companies are turning to alternative dispute resolution. We know one of the best union avoidance...
-
Article "A Leader's Journey" by Pamela Kruger Photographs by Nigel Dickson. For this discussion, let's try and unpack the key factors that led to his transformation. 1. What are your key takeaways...
-
Describe the collaborative roles of the team leader and the team coach in helping a group of people come together to form a team. Recommend strategies for Alex as team leader to use in helping to...
-
a. Complete the table with all marginal totals and cell counts. b. Calculate the following probabilities. i. For a male to be a Republican. ii. For a voter to be female. iii. For a voter to be either...
-
1. Will the Coronavirus Pandemic Make Working from Home the New Normal?" Address the following below. Define the problem described in this case. What are the management, organization, and technology...
-
Exercises 712 relate to a canonical linear programming problem with an m x n coefficient matrix A in the constraint inequality Ax b. Mark each statement True or False (T/F). Justify each answer. The...
-
In Exercises evaluate the limit, using LHpitals Rule if necessary. lim 07x cos x X
-
(a) Propose a mechanism for the acid-catalyzed reaction of salicylic acid with acetic anhydride. (b) Explain why a single drop of sulfuric acid dramatically increases the reaction rate.
-
Suppose we have some optically pure (R)-2-butyl acetate that has been "labeled" with the heavy 18O isotope at one oxygen atom as shown. (a) Draw a mechanism for the hydrolysis of this compound under...
-
(a) Explain why we speak of acidic hydrolysis of an ester as acid-catalyzed, but of basic hydrolysis as base-promoted. (b) Soap manufacturers always use base to hydrolyze fats, and never acid....
-
Show that the convexity for a zero coupon bond with m payments per year is (m) n(n + -)(1+ m m
-
Abdul Canarte , a Central Bank economist, noticed that the total group purchasing basket of goods (CPI) has gone from $149,740.00 to $344,460.00 in 8 years. With monthly compounding, what is the...
-
ABC Corporation expects sales next year to be $50,000,000. Inventory and accounts receivable (combined) will increase $8,000,000 to accommodate this sales level. The company has a profit margin of 6...

Study smarter with the SolutionInn App


