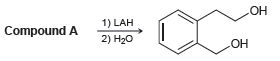
Compound A has molecular formula C 9 H 8 O 2 and exhibits a strong signal at
Question:
Transcribed Image Text:
Он 1) LAH Compound A 2) H20 ОН
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (12 reviews)
The signal at 1740 cm 1 indicates ...View the full answer

Answered By

HARSH RANJAN
Taken classes at college to graduates, Also worked as an expert to a freelancer online question-solving portal for more than 8 months with an average rating greater than 4.2 out of 5.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Compound A has molecular formula C10H10O and exhibits a strong signal at 1720 cm1 in its IR spectrum. Treatment with 1,2-ethanedithiol followed by Raney nickel affords the product shown below....
-
Compound A has molecular formula C 8 H 8 O. An IR spectrum of compound A exhibits a signal at 1680 cm -1 . The 1 H NMR spectrum of compound A exhibits a group of signals between 7.5 and 8 ppm (with a...
-
Compound A has molecular formula C 8 H 14 O 2 . Upon treatment with catalytic acid, compound. A is converted into the cyclic hemiacetal. Identify the structure of compound A. , Compound A [H*]
-
In Exercises 6780, begin by graphing the square root function, f(x) = x. Then use transformations of this graph to graph the given function. h(x)=x + 2-2
-
Here is the text book "Criminological Time Periods", Siegel explores the relationship between "crime and criminology". After reading this, provide a detailed evaluation of various sociological...
-
A vertical pole of length L is placed on top of a hill of height h. From the plain below the angles of elevation of the top and bottom of the pole are and . See Fig. 20.12. Show that Fig. 20.12. h =...
-
11. What is the rate on a synthetic FRA for a 180-day loan commencing on day 180? Suppose you are the counterparty for a borrower who uses the FRA to hedge the interest rate on a $10m loan. What...
-
On January 3, 2014, Speedway Delivery Service purchased a truck at a cost of $ 65,000. Before placing the truck in service, Speedway spent $ 4,000 painting it, $ 2,500 replacing tires, and $ 8,000...
-
The Clear Reception Antenna Company incurred the following costs while manufacturing its High Gain Long Distance Television Antenna, rated a Best Buy in Consumer Reports. Materials used in product$...
-
Describe the structure and pseudocode for an array-based implementation of an index-based list that achieves O(1) time for insertions and removals at index 0, as well as insertions and removals at...
-
Using acetonitrile (CH 3 CN) and CO 2 as your only sources of carbons, identify how you could prepare each of the following compounds: a. b. c. d.
-
Rank each set of compounds in order of increasing acidity: a. b.
-
The density of air decreases with altitude roughly as 0 e z/H , where z is the height above the Earths surface and H 8.5 km near the surface. Compute the ratio of air resistance losses for an...
-
1. Do you think that the NFL and franchise owners are meeting their obligations to employee health and safety? 2. Do you think that the NFL's and owners' responsibilities in terms of player safety...
-
Explain the term \'management\'. Also, explain briefly mission functions of management. ( b ) What are the different types of plant layout? Explain any two with neat sketches.
-
Suppose that you are considering an investment product that promises to pay $ 2 , 0 0 0 at the end of each year for the next five years. Assume that a discount rate of 1 2 % is applicable to similar...
-
Leadership Philosophy: Democratic and Transformational leadership In 700+ words ,explain how the leadership philosophy might impact an organization and how it would be beneficial.Identify what are...
-
performance and participation. The employee requirement that is met is status and recognition. The performance result is awakened drives. This model is dependent on leadership strive. It gives a...
-
Evaluate the radical expression by hand. (9-3/2)-2
-
d) For die casting processes: 1. What are the most common metals processed using die casting and discuss why other metals are not commonly die casted? 2. Which die casting machines usually have a...
-
Imagine a reaction that can replace one hydrogen atom of an alkane at random with a chlorine atom. If pentane were subjected to such a reaction, how many different compounds with the formula C5H11Cl...
-
To which compound class does each of the following compounds belong? (a) (b) C=N
-
Organic compounds can contain many different functional groups. Identify the functional groups (aside from the alkane carbons) present in acebutolol (Fig. P2.47), a drug that blocks a certain part of...
-
A stock is expected to pay a dividend of $1.50 at the end of the year (i.e., D 1 = $1.50), and it should continue to grow at a constant rate of 10% a year. If its required return is 14%, what is the...
-
The Hobby Shop has a checking account with a ledger balance of $1,700. The firm has $2,400 in uncollected deposits and $4,200 in outstanding checks. What is the amount of the disbursement float on...
-
An investment will pay you $34,000 in 11 years. If the appropriate discount rate is 6.1 percent compounded daily, what is the present value? (Use 365 days a year. Do not round intermediate...

Study smarter with the SolutionInn App


