Consider the following reaction: The following rate equation has been experimentally established for this process: Rate =
Question:
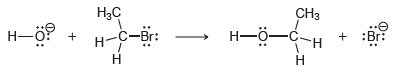
The following rate equation has been experimentally established for this process:
Rate = k [HO-] [CH3CH2Br]
The energy diagram for this process is shown below:
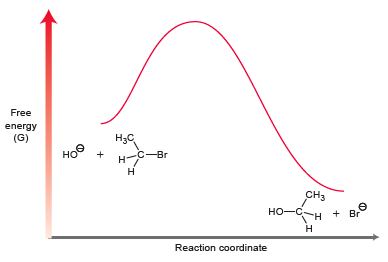
(a) Draw the curved arrows showing a mechanism for this process.
(b) Identify the two characteristic arrow-pushing patterns that are required for this mechanism.
(c) Would you expect this process to be exothermic or endothermic? Explain.
(d) Would you expect ΔSsys for this process to be positive, negative, or approximately zero?
(e) Is ΔG for this process positive or negative?
(f ) Would you expect an increase in temperature to have a significant impact on the position of equilibrium (equilibrium concentrations)? Explain.
(g) Draw the transition state of this process, and identify its location on the energy diagram.
(h) Is the transition state closer in structure to the reactants or products? Explain.
(i) Is the reaction first order or second order?
(j) How will the rate be affected if the concentration of hydroxide is doubled?
(k) Will the rate be affected by an increase in temperature?
Step by Step Answer:






