Consider the following reaction: This reaction has been determined to be second order. (a) What is the
Question:
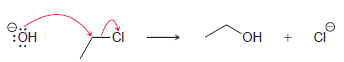
This reaction has been determined to be second order.
(a) What is the rate equation for this reaction?
(b) How will the rate be affected if the concentration of hydroxide is tripled?
(c) How will the rate be affected if the concentration of chloroethane is tripled?
(d) How will the rate be affected if the temperature is raised by 40°C?
Transcribed Image Text:
CI :ОН Он
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 92% (13 reviews)
a Rate k nucleophile substrate b The rate will be tripled because the rate is lin...View the full answer

Answered By

Fahmin Arakkal
Tutoring and Contributing expert question and answers to teachers and students.
Primarily oversees the Heat and Mass Transfer contents presented on websites and blogs.
Responsible for Creating, Editing, Updating all contents related Chemical Engineering in
latex language
4.40+
8+ Reviews
22+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the following reaction: (a) How would the rate be affected if the concentration of the alcohol is doubled? (b) How would the rate be affected if the concentration of HBr is doubled? Br - +...
-
Consider the following reaction: a) How would the rate be affected if the concentration of tertbutyl bromide is doubled? b) How would the rate be affected if the concentration of ethanol is doubled?...
-
Consider the following reaction: (a) How would the rate be affected if the concentration of the alkyl halide is doubled? (b) How would the rate be affected if the concentration of sodium cyanide is...
-
Stock X has 32% standard deviation of return. Stock Y has 32% standard deviation of return. The correlation between the returns of the two stocks is 64%. Buck's portfolio consists of equal...
-
A planar graph is a graph that can be drawn in a plane without any two edges intersecting. a. Show that neither of the graphs in Figure 9.87 is planar. b. Show that in a planar graph, there must...
-
For the following trial balance, identify the financial statement from the following list in which each title will appear (you may have more than one answer): Income Statement (IS) Statement of...
-
911 Calls A 911 service center recorded the number of calls received per hour. The random variable x represents the number of calls per hour for one week. 0 1 2 3 4 5 6 7 Xx P(x) 0.01 0.10 0.26 0.33...
-
Some of Grand Junction Corporations investment securities are classified as trading securities and some are classified as available-for-sale.The cost and market value of each category at December 31,...
-
Campbell Company reported the following data regarding the product it sells: Sales price Contribution margin ratio Fixed costs $ 60 20% $288,000 Required Use the contribution margin ratio approach...
-
According to data from the National Health and Nutrition Examination Survey, 36.5% of adult women and 26.6% of adult men are at a healthy weight. Suppose 50.52% of the adult population consists of...
-
In each group of compounds below, select the most acidic compound: (a) (b) Z Z CI CI CI
-
Consider the following substitution reaction: (a) Determine whether this reaction proceeds via an S N 1 or S N 2 process. (b) Draw the mechanism of this reaction. (c) What is the rate equation of...
-
Name and describe the two factors in particular that are crucial for enhancing self-awareness.
-
Case Study : While it might be easy to see the negative effects on the environment from car emissions or the waste we produce, fewer people think about the effects of discarded clothes on the...
-
CompanyWeek 8 Assignment - Financial Statement Analysis Overview In this assignment, you will take your work with financial statements to the next level. You will analyze financial statements similar...
-
In Exercises 9-12, assume that 100 births are randomly selected. Use subjective judgment to describe the given number of girls as (a) significantly low, (b) significantly high, or (c) neither...
-
Which of the following is not included in the cash flow statement? a. Cash from short-term investments b. Cash from operations c. Cash from the balance sheet d. Cash from capital financing Which of...
-
Case Study Chapter 13B Pharm - Saved Case Study Chapter 13 Central Nervous System Stimulants and Related Drugs Nancy has been unsuccessful in preventing migraine headaches and has been prescribed a...
-
About the size of New Jersey, Israel has seen its population soar to more than 6 million since it was established. The graphs show that by 2050, Palestinians in the West Bank, Gaza Strip, and East...
-
Privitera and Freeman (2012) constructed a scale to measure or estimate the daily fat intake of participants; the scale was called the estimated daily intake scale for fat (EDIS-F). To validate the...
-
A student wishes to record the UV spectrum of trans-stilbene, which has max = 308nm ( = 25,000), what concentration should be prepared if the desired absorbance is 0.5 at the maximum?
-
Indicate the types of transitions responsible for the absorptions of these compounds: Apax = 252 nm (e = 20,000) Aas - 325 nm ( = 180) a) A mux = 235 nm (e = 19,000) b) c) Amax = 299 nm ( = 20) d)...
-
Which of these compounds are expected to have an absorption maximum in the region of 200 to 400nm in their UV spectra? ) C-CH,CH, b) CH,CH,CH3 c) d) f) CH,CH,OCH,CH3 e) h) g)
-
In 2019, Sunland Company had a break-even point of $388,000 based on a selling price of $5 per unit and fixed costs of $155,200. In 2020, the selling price and the variable costs per unit did not...
-
11. String Conversion Given a binary string consisting of characters '0's and '1', the following operation can be performed it: Choose two adjacent characters, and replace both the characters with...
-
Consider the table shown below to answer the question posed in part a. Parts b and c are independent of the given table. Callaway Golf (ELY) Alaska Air Group (ALK) Yum! Brands (YUM) Caterpillar...

Study smarter with the SolutionInn App


