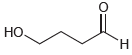
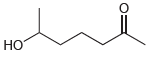
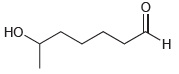
Draw the cyclic hemiacetal that is formed when each of the following bifunctional compounds is treated with
Question:
(a)
(b)
(c)
Transcribed Image Text:
но. НО H. но
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (14 reviews)
a...View the full answer

Answered By

Milan Mondal
I am milan mondal have done my Msc in physics (special astrophysics and relativity) from the University of burdwan and Bed in physical science from the same University.
From 2018 I am working as pgt physics teacher in kendriya vidyalaya no2 kharagpur ,west bengal. And also I am doing advanced physics expert in chegg.com .also I teach Bsc physics .
I love to teach physics and acience.
If you give me a chance I will give my best to you.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Two uniform bars of equal lengths I are hinged and supported as shown in Fig. 11.15. For a given vertical force P, determine the value of the horizontal force Q that would hold the system in...
-
Draw the enolate ion that is formed when each of the following compounds is treated with sodium ethoxide. In each case, draw all resonance structures of the enolate ion, and predict whether a...
-
Draw the product formed when each of the following compounds is treated with NaNO 2 and HCl: (a) (b) NH2 N.
-
Record your responses on the spreadsheet template. 2014 February 1 - Brady and Manning decide to start up a partnership. Brady brings in $10 000 cash and equipment costing $60 000, with $17 000 in...
-
Refer to the Morgan, Inc. data in Short Exercise S23-9. Last month, Morgan reported the following actual results: actual variable overhead, $10,800; actual fixed overhead, $2,770; actual production...
-
(A) Calculate the standard reaction entropy at 298.15 K for the synthesis of ammonia from its elements. (B) N 2 O 3 is an unstable oxide that readily decomposes. The standard reaction entropy for the...
-
House prices. An August 26, 2007, article in the New York Times reported that the median housing price was about $220,000. Would the mean selling price be higher, about the same, or lower? Why?...
-
Assume Adams had used his personal funds to finance his gaming activities in the Caesars casino. Under those circumstances, would he have violated any ethical or professional standards? Again, defend...
-
Developing a Master Budget for a Merchandising Organization Peyton Department Store prepares budgets quarterly. The following information is available for use in planning the second quarter budgets...
-
Lucie likes consuming candy (c) and fruit (f), and dislikes consuming plastic packaging (p), and has rational preferences over bundles (c, f, p). No matter how many units of each good she has, Lucie...
-
Identify the hydroxyaldehyde that will cyclize under acidic conditions to give the following hemiacetal: -OH
-
Consider the structures of the d aldopentoses: (a) Identify the aldopentose that is epimeric with d-arabinose at C2. (b) Identify the aldopentose that is epimeric with d-lyxose at C3. (c) Draw the...
-
A sample of 18 observations taken from a normally distributed population produced the following data: a. What is the point estimate of µ? b. Make a 99% confidence interval for µ. c. What...
-
How do we design an electromagnetic sensor?
-
What is a virtual breadboard?
-
Joe secured a loan of $13,000 four years ago from a bank for use toward his college expenses. The bank charges interest at the rate of 9%/year compounded monthly on his loan. Now that he has...
-
Answer these two questions 1 32 2 Number of Units Sold 3 4 ! Direct Material units per unit of production 5 i 6 Total Direct Materials Used 7! 8 Price Per Unit 9 10 Cost of Direct Materials 11 12 13...
-
Give an algorithm for converting a tree to its mirror. Mirror of a tree is another tree with left and right children of all non-leaf nodes interchanged. The trees below are mirrors to each other....
-
Security breaches occur with regularity in todays world, and the IRS offers tips with which a tax professional can safeguard a clients data. Find the documents (a) IRS Data Security Resource Guide...
-
Cobb Manufacturing Company uses a process cost system and average costing. The following production data is for the month of June 2011. Production Costs Work in process, beginning of the month:...
-
Determine the number of elements of unsaturation in the molecular formula C4H6. Give all nine possible structures having this formula. Remember that A double bond = one element of unsaturation A ring...
-
The preceding example shows meso-1,2-dibromo-1,2-diphenylethane reacting with iodide ion to give trans-stilbene. Show how the other diastereomer of the starting material gives a different...
-
Solved Problem 7-4 showed that the debromination of (R,R)-2,3-dibromobutane gives cis-but- 2-ene. Draw the same reaction using the (S,S) enantiomer and show that it gives the same diastereomer of the...
-
An underlying asset price is at 100, its annual volatility is 25% and the risk free interest rate is 5%. A European call option has a strike of 85 and a maturity of 40 days. Its BlackScholes price is...
-
Prescott Football Manufacturing had the following operating results for 2 0 1 9 : sales = $ 3 0 , 8 2 4 ; cost of goods sold = $ 2 1 , 9 7 4 ; depreciation expense = $ 3 , 6 0 3 ; interest expense =...
-
On January 1, 2018, Brooks Corporation exchanged $1,259,000 fair-value consideration for all of the outstanding voting stock of Chandler, Inc. At the acquisition date, Chandler had a book value equal...

Study smarter with the SolutionInn App


