Each of the following alcohols can be prepared via reduction of a ketone or aldehyde. In each
Question:
a.
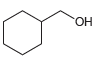
b.
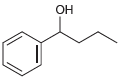
c.
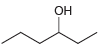
d.
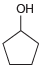
Transcribed Image Text:
Но. Он ОН
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (14 reviews)
a b c...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Show how each of the following alcohols can be made from a Grignard reagent and a carbonyl compound: a. b. 0 0
-
Each of the following alcohols has been subjected to acidcatalyzed dehydration and yields a mixture of two isomeric alkenes. Identify the two alkenes in each case, and predict which one is the major...
-
Each of the following alcohols has been subjected to acidcatalyzed dehydration and yields a mixture of two isomeric alkenes. Identify the two alkenes in each case, and predict which one is the major...
-
The RRR Company has a target current ratio of 2.4. Presently, the current ratio is 3.3 based on current assets of $6,567,000. If RRR expands its inventory using short- term liabilities (maturities...
-
On January 10, VW agrees to import auto parts worth $7 million from the US. The parts will be delivered on March 4 and are payable immediately in dollars. VW decides to hedge its dollar exposure by...
-
Find the curvature K of the plane curve at the given value of the parameter. r(t) = ti + ti - + =j, t = 1
-
2 You and three college friends have decided to launch an online business selling clothes college students wearT-shirts, shorts, sweats, and so on. You plan to use Facebook ads. What likes or...
-
Journalize the following transactions in the accounts of Pro Medical Co., a medical equipment company that uses the direct write-off method of accounting for uncollectible receivables: Jan. 30. Sold...
-
Apollo is a local hospital that offers two types of procedures. Currently, Apollo uses a simple system to calculate the overhead cost per treatment. This is done by taking the total overhead cost and...
-
Harry is a trader selling goods on credit. His financial year ends on 31 December. The balances on his books on 1 January 203 included the following: $ Provision for doubtful debts...
-
Starting with 1-butanol, show the reagents you would use to prepare each of the following compounds. a. b. c. d. e. H. .
-
Each of the following alcohols can be prepared via reduction of a ketone or aldehyde. In each case, identify the aldehyde or ketone that would be required. a. b. c. d. .
-
Are there any locations that stand out as unusual and that might therefore warrant special study? Explain Melanoma Mortality. For Exercises, refer to Figure 3.26, which shows the female mortality...
-
How can you filter on a particular data field in Cognos Analytics? 0 / 1 point Type in the name of the field in the 'Filters' area at the top of the page. Drag the data field to the 'All tabs' area...
-
Project management is fundamental to project success and includes a number of critical success factors including support of top management, use of effective communication channels and rapid feedback,...
-
BIO 189: what are some of the ethical issues regarding his results? why were his results rejected by the scientific community? choose two components below that were either flawed or completely absent...
-
REQUIRED: Prepare Cost of Production per department. Problem 5 ABM Company uses two departments to produce a product. The following data were taken from the books for the month of January, 2019....
-
Can you describe how Toyota responds to changes in market fluctuations, and plot the supply and demand curves indicating managerial economic principles (i.e. price ceilings/floors, shortage/surplus,...
-
If f(3) = 2 and g(3) = 5, (f + g)(3)
-
Assume a simple Keynesian depression economy with a multiplier of 4 and an initial equilibrium income of $3,000. Saving and investment equal $400, and assume full employment income is $4,000. a. What...
-
Isoleucine and threonine are the only two amino acids with two chirality centers. Assign R or S configuration to the methyl-bearing carbon atom ofisoleucine.
-
Is the following structure a D amino acid or an L amino acid? Identifyit.
-
Give the sequence of the following tetrapeptide:
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...

Study smarter with the SolutionInn App


