For each of the following alkenes, assign the configuration of the double bond as either E or
Question:
a.
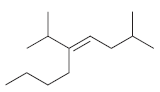
b.
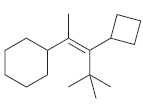
c.
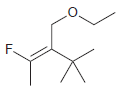
d.
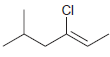
Transcribed Image Text:
F.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 90% (20 reviews)
a...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The second-order rate constant (in units of M-1 s-1) for acid-catalyzed hydration at 25°C is given for each of the following alkenes: a. Calculate the relative rates of hydration of the alkenes....
-
Name each of the following alkenes or alkynes. a. CH2 = CH-CH2-CH3 b. c. d. e. f. g. CH3 C-CH-CH3 CH3 CH CH3 CH3CH2CH CH CH CH CH3 CH, C-CH-CH CH, CH2-CH, CH3 CH2CHs CH, CH2CH3 CH3 C C-CH CH3 CH3
-
Explain why each of the following alkenes is stable or unstable. (a) 1,2-dimethylcyclopentene (b) trans-1,2-dimethylcyclopentene (c) trans-3,4-dimethylcyclopentene (d) trans-1,2-dimethylcyclodecene...
-
In your opinion, was Saks' zero tolerance policy for employee theft reasonable? Was the policy likely cost-effective? Defend your answers.
-
What happens when a person has too much money? What about when an economy has too much money?
-
What are some quantitative decision sciences that are drawn upon by business analytics?
-
What is PV? AppendixLO1
-
Following is a list of advantages and disadvantages of the corporate form of business. 1. Ownership and management are separated. 2. Has continuous life. 3. Transfer of ownership is easy. 4....
-
PLEASE SHOW ALL WORK! THANK YOU Exercise 17-04 Your answer is partially correct. Try again. On January 1, 2020, Oriole Company purchased 12% bonds, having a maturity value of $316,000 for...
-
River Cruises' management now understands that the trade-off theory of optimal capital structure implies managers will increase debt as long as the value of additional interest tax shields exceeds...
-
Which of the following best describes the efficiency of monopolistically competitive firms? a. Allocatively efficient by productively inefficient. b. Allocatively inefficient but productively...
-
Draw the mechanism and predict the product of the following reaction. In this case, H 3 O + must be used as a proton source instead of water. Explain why. 1) xs MeMgBr 2) H*
-
Four RAM memories are connected to CPU busses as shown here. Assume that the following RAM component is available.module SRAM(cs-b, we-b, oe-b, address, data);input cs-b,we-b,oe-b;input[14:0]...
-
Haley Romeros had just been appointed vice president of the Rocky Mountain Region of the Bank Services Corporation (BSC). The company provides check processing services for small banks. The banks...
-
Draw a simple but complete hydraulic circuit diagram to drive two actuators, one of which must be connected to a pressure reducing valve to control its pressure because of the delicacy of the task...
-
1. What specific skills would a person have to be a successful director for a parks and recreation position? 2. What experience would a person have working with an elected board for parks and...
-
Required labor time per unit ( hours ) Maximum demand ( units ) Contribution margin per unit Product M 2 6 , 5 0 0 $ 5 . 0 0 Product N 3 8 , 0 0 0 $ 5 . 7 0 If Bush uses the most effective approach...
-
ansewr pls Repeat Exercise 5.8.1 using (a) one rectangle; (b) four rectangles. Data From Exercise 5.8.1 A square plate size \(100 \mathrm{~cm} \times 100 \mathrm{~cm}\) is subjected to an isothermal...
-
Find f -1 (x). f(x) = 6 - 3(2x-4)
-
Describe a group you belong or have belonged discuss the stages of group development and suggest how to improve the group effectiveness by using the group development model.
-
One of the two chair structures of cis-1-chloro-3-methylcyclohexane is more stable than the other by 15.5kJ/mol (3.7kcal/mol). Which is it? What is the energy cost of a 1, 3-diaxial interaction...
-
The German chemist J. Bredt proposed in 1935 that bicycloalkenes such as 1-norbornene, which have a double bond to the bridgehead carbon, are too strained to exist. Make a molecular model of...
-
Tell whether each of the following substituents on a steroid is axial or equatorial. (A substituent that is ?up? is on the top face of the molecule as drawn, and a substituent that is ?down? is on...
-
Ventaz Corp manufactures small windows for back yard sheds. Historically, its demand has ranged from 30 to 50 windows per day with an average of 4646. Alex is one of the production workers and he...
-
Which of the following statements is not true regarding the $500 credit for dependent other than a qualifying child credit. Cannot be claimed on the same tax return if the child tax credit is also...
-
Grind Co. is considering replacing an existing machine. The new machine is expected to reduce labor costs by $127,000 per year for 5 years. Depreciation on the new machine is $57,000 compared with...

Study smarter with the SolutionInn App


