For each of the following carbocations determine if it will rearrange, and if so, draw the carbocation
Question:
a.
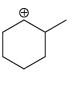
b.
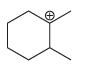
c.
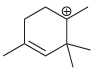
d.

e.

f.

g.
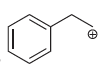
h.

Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (8 reviews)
a b This carbocation is tertiary and will not rearrange c d Th...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Write a second resonance structure for each of the following carbocations: (a) (b) (c) CH3CH CHH2 ( 3)2 4
-
Each of the following carbocations has the potential to rearrange to a more stable one. Write the structure of the rearranged carbocation. (a) CH3CH2CH2+ (b) (CH3)2CHC_HCH3 (c) (CH3)3CCHCH3 (d)...
-
Each of the following carbocations can rearrange to a more stable ion. Propose structures for the likely rearrangement products. H, (a) CH3CH2CH2CH2* (b) CH3CHCHCH3 CH CH CH2* (c)
-
Evaluate the geometric series or state that it diverges. 8 00 k=0 2k 75
-
The premium on a call option on the market index with an exercise price of 100 is $1.90 when originally purchased. After 2 months the position is closed and the index spot price is 102. If interest...
-
What was the change in Globals book value of equity from 2018 to 2019 according to Table 2.1? Does this imply that the market price of Globals shares increased in 2019? Explain. Assets Current Assets...
-
Explain actual overhead rate and predetermined overhead rate
-
Fun Ltd., a Texas company, is an expert in the assembly of a variety of video games consoles and they also offer repair parts for these systems. The products range from small handheld consoles that...
-
Laura Corbins regular hourly wage rate is $20, and she receives an hourly rate of $30 for work in excess of 40 hours. During a January pay period, Laura works 45 hours. Lauras federal income tax...
-
Review The Power of Good Design and select three of the ten principles noted for good design. Next in R, utilize these three principles in a problem that you will solve. First note the problem to...
-
Draw the curved arrows that accomplish each of the following transformations: a. b. c. H. H.
-
In each of the following cases compare the bonds identified with red arrows, and determine which bond you would expect to have the largest bond dissociation energy: a. b. CI .F Br
-
An RL circuit is shown in Figure PI 1.21. (a) Select the two stable variables and obtain the vector differential equation where the output is v0 (t). (b) Determine whether the state variables are...
-
A farmer has an acre of specialty vegetables and is preparing for the summer harvest. Historically, this acre has yielded an average of 2,100 lbs of product with a standard deviation of 950 lbs. A...
-
Solve 3x 82+22 = (4).
-
(c) Compute EVPI and EVSI (in thousands of dollars). (Round your answers to one decimal place.) EVPI $ 3.6 EVSI $ 3.6 Xthousand x thousand Discuss whether the firm should consider a consulting expert...
-
Question 9 (1 point) If the common law requires employees of a bar establishment to monitor a potentially intoxicated patron and to possibly make an effort to intervene if there is an indication the...
-
B. A velocity potential is given by the equation: Q = x-y 3. (10 pts) Short answer, What special characteristics of the velocity potential make it very useful in identifying a type of flow and...
-
In Exercises 7192, find and simplify the difference quotient f(x)=x - 1
-
Give codons for the following amino acids: (a) Th (b) Asp (c) Thr
-
When pseudoionone is treated with BF3 in acetic acid, ring closure takes place and α-and β-ionone are produced. This is the next step in the vitamin A synthesis. (a) Write...
-
(a) Write resonance structures for the anion of acetonitrile that account for its being much more acidic than ethane. (b) Give a step-by-step mechanism for the condensation of benzaldehyde with...
-
Starting with ketones and aldehydes of your choice, outline a directed aldol synthesis of each of the following using lithium enolates: (a) (b) (c) O OH CGH5 O OH
-
What is Coke's average ownership percentage in its equity method investments? Goodwill is 7000 Calculate the firm's current ratio (current assets/current liabilities). Calculate the current ratio...
-
John has to choose between Project A and Project B, which are mutually exclusive. Project A has an initial cost of $30,000 and an internal rate of return of 16 percent. Project B has an initial cost...
-
Complete the table below, for the above transactions

Study smarter with the SolutionInn App


