For each of the following compounds, determine the position that is most likely to be the site
Question:
(a)
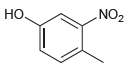
(b)
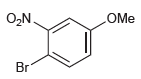
(c)
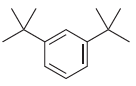
(d)
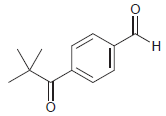
(e)
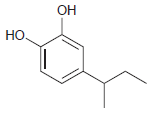
Transcribed Image Text:
Но. NO2 O,N OMe Br
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (10 reviews)
a b...View the full answer

Answered By

BRIAN MUSINGA
I possess a Bachelors of Commerce degree(Marketing option) and am currently undertaking an MBA in marketing. I believe that I possess the required knowledge and skills to tutor in the subject named. I have also written numerous research academic papers much to the satisfaction of clients and my professors.
5.00+
2+ Reviews
17+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
For each of the following compounds, compare the two indicated protons and determine whether they are enantiotopic, homotopic, or diastereotopic: (a) (b) (c) (d) (e) (f) (g) (h) (i) (j) (k) (l) (m)...
-
For each of the following compounds, determine whether the two protons shown in red are homotopic, enantiotopic, or diastereotopic: (a) (b) (c) (d) (e) Discuss. OMe . CI H,
-
For each of the following compounds determine which (if any) lone pairs are participating in aromaticity. a. b. c. d. e. f. g. h. :N-H
-
Consider the plane stress inhomogeneous case with only variation in elastic modulus given by E = E(y) = 1/ (Ay + B). Further assume that the Airy function depends only on y, = (y). Show that...
-
Describe how a small business owner could use the following sources of a competitive advantage: "niche-picking," "entertailing," emphasizing their uniqueness, connecting with their customers,...
-
Use a calculator to solve the given equation. 4(3 x ) = 5
-
E 20-9 Preparation of fund balance sheet A general ledger trial balance at June 30, 2013, for Millar City is as follows: Debits Credits Cash $12,000 Taxes receivable 30,000 Allowance for...
-
AIG, which received more than $170,000,000 in taxpayer bailout money from the U.S. Treasury, planned to pay $165,000,000 in bonuses to its executives in 2009. Requirement 1. Suppose you were one of...
-
Kareen Ltd is a retail store specialising in home accessories stores and ornaments. The following transactions include GST. Oct.4 Purchased inventory on credit terms of 3/10, net eom, $9 900. 8...
-
What is the solution of the recursive equation T (n) = 0.015625T +n?? (1/4) a) O(n') b) O(n' lg n) c) O(n* Ign) d) O(7*)
-
When 2,4-dibromo-3-methyltoluene is treated with bromine in the presence of iron (Fe), a compound with molecular formula C8H7Br3 is obtained. Identify the structure of this product.
-
For the reaction C(graphite) + H 2 O(g) CO(g) + H 2 (g), H o R =131.28 kJ mol -1 at 298.15 K. Use the values of C P,m at 298.15 K in the data tables to calculate H R at 125.0C.
-
Hiram Foster, Inc., has two departments: luggage and acces sories. Fosters accountant prepares the adjusted trial balance shown on page 988 at the end of the fiscal year, after all adjustments,...
-
2. Ten bars of a certain quality are tested for their diameters. The results are given below. Test the hypothesis at a 95% level of confidence that the mean diameter of the bars produced by the...
-
Write out the state of the list while being sorted using the bubble sort algorithm. 5 8 3 6 9 5 Java code is required.
-
f(x) = 2 X x + 25 X local maximum value local minimum value Need Help? Read It
-
Airbed and Breakfast (428 words): The Startup Story of AirbnbBrian and Joe were flat mates in downtown San Francisco. In mid-2007, IDSA conference crowded downtown, filled all hotel rooms, and drove...
-
# We are testing Null: = 100 against Alternative: 100 using a sample size of 15. The critical values for t for a = .10 are
-
In Exercises 130133, write the equation of a rational function f(x) = p(x)/q(x) having the indicated properties, in which the degrees of p and q are as small as possible. More than one correct...
-
In July 2013, cnet.com listed the battery life (in hours) and luminous intensity (i. e., screen brightness, in cd/m2) for a sample of tablet computers. We want to know if screen brightness is...
-
Use Figure 12.2 to answer the following question. How does the energy of X-rays compare with that of blue light (greater or smaller)? Figure 12.2 1023 02cosmic rays 1oto 10g 108 107 106 10* 104 10...
-
(a) You have found in the laboratory two liquids, C and D, in unlabeled bottles. You suspect that one is deuterated chloroform (CDC13) and the other is ordinary chloroform (CHC13). Unfortunately, the...
-
(a) You have found in the laboratory two liquids, C and D, in unlabeled bottles. You suspect that one is deuterated chloroform (CDC13) and the other is ordinary chloroform (CHC13). Unfortunately, the...
-
If John invested $20,000 in a stock paying annual qualifying dividends equal to 4% of his investment, what would the value of his investment be 5 years from now? Assume Johns marginal ordinary tax...
-
help asap please!
-
Please, help asap! I have one day. Feedback will be given. & show some work. [in Excel] For the final project you will need you to create a spreadsheet /proforma of the cash flows from a property....

Study smarter with the SolutionInn App


