For each of the following pairs of compounds, determine the relationship between the two compounds: a. b.
Question:
a.
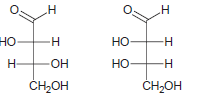
b.
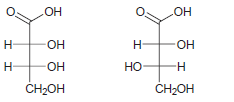
c.
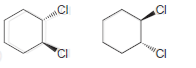
d.
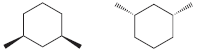
e.
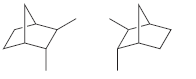
f.
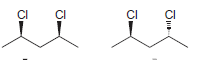
g.
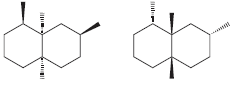
h.
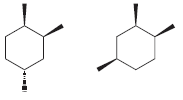
i.
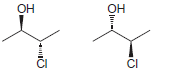
j.
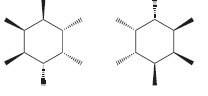
k.
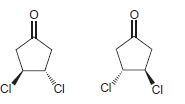
l.
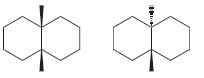
Transcribed Image Text:
н н но- —н Но— —н но- Н -ОН -н CH-он ČH2OH он но" н- -ОН н- он Н ОН Но- н CH-ОН ČH2OH エ エ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 46% (13 reviews)
a Diastereomers b Diastereomers c En...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Each of the following pairs of compounds undergoes a Bronsted acid-base reaction for which the equilibrium lies to the right. Give the products of each reaction, and identify the acid, the base, the...
-
In each of the following pairs of compounds one is chiral and the other is achiral. Identify each compound as chiral or achiral, as appropriate. a. b. c. d. CICH CHCH OH and HOCH CHCH,OH CI OH CH,CH...
-
In each of the following pairs of compounds choose which one will react faster with the indicated reagent, and write a chemical equation for the faster reaction: (a) Toluene or chlorobenzene with a...
-
Based on Exhibit 1, the expected future value of Bond I at maturity is closest to: A. 98.80. B. 103.74. C. 105.00. Lena Liecken is a senior bond analyst at Taurus Investment Management. Kristel...
-
Why are pro forma financial statements important to the financial planning process?
-
What is middleware?
-
A firm experienced abnormal scrap and collected data to see which parts were causing the problem with the following results: part A$5720, part B$10,250, Part C$820, Part D$1130, Part F$700. Complete...
-
Waterways Corporation is a private corporation formed for the purpose of providing the products and the services needed to irrigate farms, parks, commercial projects, and private lawns. It has a...
-
Assume a company provided the following information: High activity level (September) Low activity level (May) Patient-Days 3,500 2,500 Maintenance Cost $ 10,400 $ 9,200 Using the high-low method, the...
-
Enter the transactions for Weeks 1 5 into the appropriate Journal for the month of June 2022. Next, post all the Journal transactions to the ledger accounts as specified in the Account System...
-
Why is the EOQ model described as robust?
-
Identify the stronger nucleophile: (a) NaSH vs. H 2 S (b) Sodium hydroxide vs. water (c) Methoxide dissolved in methanol vs. methoxide dissolved in DMSO
-
Following are time-series data for eight different periods. Use exponential smoothing to forecast the values for periods 3 through 8.Use the value for the first period as the forecast for the second...
-
How could civil engineers contribute to space debris management and cleanup?
-
In what ways can the principles of resilient infrastructure be applied to design urban systems capable of withstanding natural disasters, and how do these principles contribute to the overall safety...
-
What are local variables and global variables in Python?
-
When to use a tuple vs list vs dictionary in Python? Explain some benefits of Python
-
What is Lambda Functions in Python? How do I modify a string in python?
-
In Exercises 4556, use transformations of f(x) = 1/x or f(x) = 1/x 2 to graph each rational function. h(x) || 1 (x - 3) + 1
-
With your classmates, form small teams of skunkworks. Your task is to identify an innovation that you think would benefit your school, college, or university, and to outline an action plan for...
-
Ethyl triflate is much more reactive than ethyl mesylate toward nucleophiles in SN2 reactions. (a) Give the structures of all of the products formed when each compound reacts with potassium iodide in...
-
How many grams of CrO3, are required to oxidize 10 g of 2-heptanol to the colresponding ketone?
-
How many grams of CrO3, are required to oxidize 10 g of 2-heptanol to the colresponding ketone?
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...

Study smarter with the SolutionInn App


