How many signals do you expect in the 13 C NMR spectrum of each of the following
Question:
a.
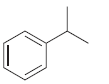
b.
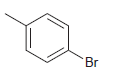
c.
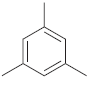
d.
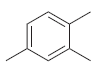
Transcribed Image Text:
Br
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)
a 6...View the full answer

Answered By

Vijesh J
My passion to become a tutor is a lifetime milestone. Being a finance and marketing professional with hands-on experience in wealth management, portfolio management, team handling and actively contributing in promoting the company. Highly talented in managing and educating students in most attractive ways were students get involved. I will always give perfection to my works. Time is the most important for the works and I provide every answer on time without a delay. I will proofread each and every work and will deliver a with more perfection.
4.70+
5+ Reviews
15+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
How many signals do you expect in the 1 H NMR spectrum of each of the following compounds: (a) (b) (c) Geraniol Isolated from roses and used in perfumes . NH2 Dopamine A neurotransmitter that is...
-
Consider the following compound: (a) How many signals do you expect in the 1 H NMR spectrum of this compound? (b) Rank the protons in terms of increasing chemical shift. (c) How many signals do you...
-
Dimethylformamide (DMF) is a common solvent: (a) The 1 H NMR spectrum of DMF exhibits three signals. Upon treatment with excess LAH followed by water, DMF is converted into a new compound that...
-
Solve each system. If a system is inconsistent or has dependent equations, say so. -5x + 2y = -4 6x + 3y = -6
-
Assume the role of a social science researcher in charge of developing a new crime prevention program targeted toward juveniles in the K-12 school system. Review current crime prevention programs...
-
Evaluate exactly the given expressions if possible. cos 1 [cos(/4)]
-
15. What 8-quarter dollar annuity is equivalent to an 8-quarter annuity of =C1?
-
A large automobile insurance company selected samples of single and married male policyholders and recorded the number who made an insurance claim over the preceding three-year period. a. Use = .05....
-
Effect of transactions on cash flows State the effect (cash receipt or cash payment and amount) of each of the following transactions, considered individually, on cash flows: a. Retired $190,000 of...
-
In the code below, three processes are competing for six resources labeled A to F. a. Using a resource allocation graph, show the possibility of a deadlock in this implementation. b. Modify the order...
-
How would you distinguish between each pair of compounds in Problem 15.29 using IR spectroscopy? Problem 15.29 a. b. OH HO. HO m/z = 126.0315 m/z = 126.1404
-
Draw a Frost circle for the following cation, and explain the source of instability of this cation.
-
A model steam engine of 1.00-kg mass pulls eight cars of 1.00-kg mass each. The cars start at rest and reach a velocity of 3.00 m/s in a time of 3.00 s while moving a distance of 4.50 m. During that...
-
Briefly, discuss the use of survey research in exploratory, descriptive, explanatory, and evaluation studies. Using a criminal justice example select one type of research study and develop one...
-
Medical Helicopters In a study of helicopter usage and patient survival, results were obtained from 47,637 patients transported by helicopter and 111,874 patients transported by ground (based on data...
-
Woodland Hills Company reported income before taxes (pretax financial income) in its income statement of $60,000. Among the items included in the computation of pretax financial income were the...
-
cest Shouldice Hospital in Canada is widely known for one thing-hernia repair! In fact, that is the only operation it performs, and it performs a great many of them. Over the past two decades this...
-
The activation energy for the gas phase decomposition of isobutyl bromide is 211 kJ. (CH3)2CHCH2 Br (CH3)2C=CH2+ HBr The rate constant at 676 K is 5.73 x 10-4 s. The rate constant will be 0.00647 s...
-
Graph y = f(x). You may want to use division, factoring, or transformations as an aid. Show all asymptotes and "holes." f(x)= 4x + 4x + 1 2x + 1
-
Per Bag Direct materials: 25 pounds of CWhiz-2000 @ $0.08/lb. = $ 2.00 Direct labor: 0.05 hour @ $32.00/hr. = $ 1.60 The company manufactured 100,000 bags of Cheese-Be-Good in December and used...
-
Which of the following alkenes would yield the same alcohol from either oxymercuration-reduction or hydroboration--oxidation, and which would give different alcohols? Explain. (a) cis-2-butene (b)...
-
Give the products (if any) expected from the treatment of each of the following compounds with ozone followed by dimethyl sulfide (a) (b) 2-methylpentane CH2
-
Give the products (if any) expected when the compounds in Problem 5.13 are treated with ozone followed by aqueous hydrogen peroxide.
-
Suppose First Fidelity Bank engaged in the following transactions: (Click the icon to view the transactions.) Journalize the 2018 and 2019 transactions on First Fidelity's books. Explanations are not...
-
Financial data for Joel de Paris, Inc., for last year follow: Joel de Paris, Inc. Balance Sheet Beginning Balance Ending Balance Assets Cash Accounts receivable Inventory Plant and equipment, net...
-
Supply costs at Coulthard Corporation's chain of gyms are listed below: March April May June July August September October November Client-Visits 11,666 11,462 11,994 13,900 11,726 11, 212 12,006...

Study smarter with the SolutionInn App


