How many signals do you expect in the 1 H NMR spectrum of each of the following
Question:
(a)
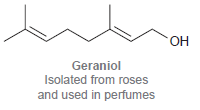
(b)
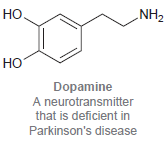
(c)
Transcribed Image Text:
ОН Geraniol Isolated from roses and used in perfumes но. NH2 но Dopamine A neurotransmitter that is deficient in Parkinson's disease
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (10 reviews)
a Ni...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
How many signals do you expect in the 13 C NMR spectrum of each of the following compounds? a. b. c. d. Br
-
Consider the following compound: (a) How many signals do you expect in the 1 H NMR spectrum of this compound? (b) Rank the protons in terms of increasing chemical shift. (c) How many signals do you...
-
Dimethylformamide (DMF) is a common solvent: (a) The 1 H NMR spectrum of DMF exhibits three signals. Upon treatment with excess LAH followed by water, DMF is converted into a new compound that...
-
discuss case study a bout remote analysis during covid 1 9 - 1 9 virus
-
What are two examples of industries that could benefit from IoM?
-
Create a sunburst chart for the regions, items, and units sold in the Excel file Store and Regional Sales Database.
-
Explain the purposes of each step of the new-product process.
-
In another version of the "Giant Swing" (see Exercise 5.52), the seat is connected to two cables as shown in Fig. 5.58, one of which is horizontal. The seat swings in a h0ri2ontal circle at a rate of...
-
Question 4 of 5 -/5 View Policies Current Attempt in Progress Restate the following income statement for a merchandising company in contribution format. Sales ($ 20 per unit) $ 20,000 Less cost of...
-
The Brasher doubloon, which was featured in the plot of the Raymond Chandler novel, The High Window, was sold at auction in 2018 for a reported $5.5 million. The coin had a face value of $15 when it...
-
Daniel Barnes, financial manager of New York Fuels (NYF), a heating oil distributor, is concerned about the companys working capital policy, and he is considering three alternative policies: (1) a...
-
Rank the signals of the following compound in terms of increasing chemical shift. Identify the proton(s) giving rise to each signal: CI. .
-
Gray Company leased a delivery truck on January 1, 2012. The lease requires annual payments of $7,500 for seven years at a 12% rate of interest payable at the end of each year. The company classifyes...
-
Convert the following information into: a) a semantic net b) a frame-based representation A Ford is a type of car. Bob owns two cars. Bob parks his car at home.His house is in California, which is a...
-
Visit www.pearsonglobaleditions.com/malhotra to read the video case and view the accompanying video. Marriott: Marketing Research Leads to Expanded Offerings highlights Marriotts success in using...
-
The water level in a tank is \(20 \mathrm{~m}\) above the ground. A hose is connected to the bottom of the tank, and the nozzle at the end of the hose is pointed straight up. The tank cover is...
-
A simple experiment has long been used to demonstrate how negative pressure prevents water from being spilled out of an inverted glass. A glass that is fully filled by water and covered with a thin...
-
A golf ball is hit on a level fairway. When it lands, its velocity vector has rotated through an angle of 90. What was the launch angle of the golf ball? Pyo By Dyz =0 Uso Range R x max dya
-
State the inverse action or actions. Climbing up a ladder
-
The Home Depot is the leading retailer in the home improvement industry and one of the 10largest retailers in the United States. The company included the following on its January 29, 2012, balance...
-
In humans, the final product of purine degradation from DNA is uric acid, pKa = 5.61, which is excreted in the urine. What is the percent dissociation of uric acid in urine at a typical pH = 6.0? Why...
-
Shown here are some pKa data for simple dibasic acids. How can you account for the fact that the difference between the first and second ionization constants decreases with increasing distance...
-
Predict the product of the reaction of p-methyl benzoic acid with each of the following: (a) LiAlH 4 , then H 3 O + (b) N-Bromosuccinimide in CCl 4 (c) CH 3 MgBr in ether, then H 3 O + (d) KMnO 4 , H...
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...

Study smarter with the SolutionInn App


