Identify the reagents you might use to achieve each of the following transformations: Br Br , En
Question:
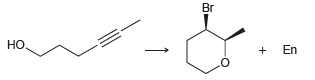
Transcribed Image Text:
Br Br но, En
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 56% (16 reviews)
HO H Lindlars Catalyst HO Note Y...View the full answer

Answered By

Ajeet Singh
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions.
I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life.
I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge.
I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields.
Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a teacher. I have done plenty of studying and lots of learning on many exciting and challenging topics. All these experiences have influenced my decision to take on the teaching role in various capacities. As a tutor, I am looking forward to getting to understand your needs and helping you achieve your academic goals. I'm highly flexible and contactable. I am available to work on short notice since I only prefer to work with very small and select groups of students.
4.90+
7+ Reviews
15+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Identify the reagents you would use to achieve each of the following transformations: (a) (b) (c) (d) (e) OTs `CN
-
Identify the reagents you would use to achieve each of the following transformations: a) Convert tert-butyl bromide into a primary alkyl halide b) Convert 2-bromopropane into 1-bromopropane
-
Identify the reagents you would use to convert 2-bromo-2-methylbutane into 3-methyl-1-butyne.
-
Name the following compounds, and indicate whether each is a reducing sugar or a nonreducing sugar: a. b. c. d. CH,OH HO OCH2CH2CH3 OH HO CH2OH OCH3 - HOCH OCH2CH3 CH OH OH OH
-
Suppose we want to add an extra operation, deunion, which undoes the last union operation that has not been already undone. a. Show that if we do union-by-height and finds without path compression,...
-
At December 31, 2021, Landy Products has cash of $24,000, receivables of $18,000, and inventory of $80,000. The companys equipment totals $182,000. Landy owes accounts payable of $22,000 and...
-
7 Suponga que alguien le dice: La nica medida real de un vendedor es el volumen de ventas producido. Qu le respondera?
-
Zigs Industries had the following operating results for 2011: sales = $27,360; cost of goods sold = $19,260; depreciation expense = $4,860; interest expense = $2,190; dividends paid = $1,560. At the...
-
1. 2. 3. Zorro Enterprises began operations in 2017 and has been using a LIFO valuation method for inventory. For a justifiable reason, Zorro has decided to switch to the FIFO method in 2020. The...
-
A thermocouple junction of area A, mass m, heat capacity C, and emissivity e is located in a furnace that normally is at TC. At these temperatures convective and conductive heat transfer to the...
-
The economic tool that proves the value of an expensive college education is a. Production possibilities. b. The yield curve. c. Supply and demand. d. Present value.
-
The cost of educating a college student a. Is less than the cost of educating a high school student because college classes are generally large. b. Is equal to the cost of educating a high school...
-
At the height of the COVID pandemic, the government of Greece issued a 2 percent annual coupon bond maturing in seven years. 1. If the observed YTM at issuance was 2.00 percent, what was the issuance...
-
In 2024, the Westgate Construction Company entered into a contract to construct a road for Santa Clara County for $10,000,000. The road was completed in 2026. Information related to the contract is...
-
Briefly describe the case you have chosen. Categorize the social worker's experience as vicarious trauma, compassion fatigue, or burnout. Provide justification. Identify the social worker's score on...
-
Given f(x) below, find f'(x). f(x) = = m 5z In (2) et dt
-
Olsen & Alain, CPAs (O&A) performed the audit of Rocky Point Brewery (RPB), a public company in 20X1 and 20X2. In 20X2, O&A also performed tax services for the company. Which statement best describes...
-
Exercise 9-4 (Algo) Prepare a Flexible Budget Performance Report [LO9-4] Vulcan Flyovers offers scenic overflights of Mount Saint Helens, the volcano in Washington State that explosively erupted in...
-
In Exercises 110, approximate each number using a calculator. Round your answer to three decimal places. 3 5
-
The maximum pressure that can be developed for a certain fluid power cylinder is 15.0 MPa. Compute the required diameter for the piston if the cylinder must exert a force of 30 kN.
-
Organic phosphate groups occur commonly in biological molecules. Calculate formal charges on the four O atoms in the methyl phosphatedianion. 2- :0: H-C-0-P-0: Methyl phosphate :0:
-
Draw the indicated number of resonance forms for each of the following species: (a) The methyl phosphate anion, CH3OPO32- (3) (b) The nitrate anion, NO3- (3) (c) The allyl cation, H2C = CH ? CH2+ (2)...
-
Nitric acid (HNO3) reacts with ammonia (NH3) to yield ammonium nitrate. Write the reaction, and identify the acid, the base, the conjugate acid product, and the conjugate base product.
-
Break-Even Sales and Sales to Realize Income from Operations For the current year ending October 31, Yentling Company expects fixed costs of $537,600, a unit variable cost of $50, and a unit selling...
-
You buy a stock for $35 per share. One year later you receive a dividend of $3.50 per share and sell the stock for $30 per share. What is your total rate of return on this investment? What is your...
-
Filippucci Company used a budgeted indirect-cost rate for its manufacturing operations, the amount allocated ($200,000) is different from the actual amount incurred ($225,000). Ending balances in the...

Study smarter with the SolutionInn App


