In each compound below, two protons are clearly identified. Determine which of the two protons is more
Question:
(a)
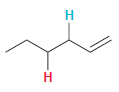
(b)
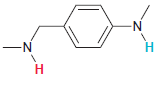
(c)
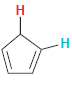
(d)
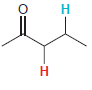
(e)
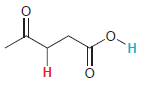
(f)
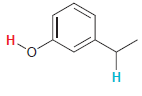
Transcribed Image Text:
Н Н -N Н Н
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (12 reviews)
a ...View the full answer

Answered By

Shem Ongek
I am a professional who has the highest levels of self-motivation. Additionally, I am always angled at ensuring that my clients get the best of the quality work possible within the deadline. Additionally, I write high quality business papers, generate quality feedback with more focus being on the accounting analysis. I additionally have helped various students here in the past with their research papers which made them move from the C grade to an A-grade. You can trust me 100% with your work and for sure I will handle your papers as if it were my assignment. That is the kind of professionalism that I swore to operate within. I think when rating the quality of my work, 98% of the students I work for always come back with more work which therefore makes me to be just the right person to handle your paper.
4.80+
174+ Reviews
426+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
PLS In the Loan Calculator worksheet, the formula in cell D8 should calculate the total cost of the loan. 109 2/3 27. Bao wants t atas Table Get External Data Refresh All Stocks Geogra... L Sort...
-
Which of the following compounds is more easily decarboxylated? CH2CH2 or
-
Which of the following compounds is more likely to exhibit activity as a tranquilizer? CH3 CH CH O or CH C
-
In Exercises, find the limit. x-4 lim x-00x + 1
-
Suppose the risk free rate of return is 6%, maturity risk premium is 2%, inflation premium is 4%, the default risk on similar debt is 3%, and the liquidity premium is 2%. What is the nominal interest...
-
Sketch the graph of the level surface (x, y, z) = c at the given value of c. (x, y, z) = x - y + z, c = 2
-
Errors in surveys. A college chooses an SRS of 100 students from the registrars list of all undergraduates to interview about student life. If it selected two SRSs of 100 students at the same time,...
-
Brinkley Company, which began operations on January 3, 2015, had the following subsequent transactions and events in its long-term investments. 2015 Jan. 5 Brinkley purchased 20,000 shares (25% of...
-
Why is it important to classify costs into a cost hierarchy? A. It is important to classify costs into a cost hierarchy because it allows managers to evaluate the importance of costs in the...
-
Refer to the table in Problem 3.8. In Problem 3.8, the City Commission of Nashville has decided to build a botanical garden and picnic area in the heart of the city for the recreation of its...
-
Nitrogen and sulfur are neither in the same row nor in the same column of the periodic table. Nevertheless, you should be able to identify which proton below is more acidic. Explain your choice: .S....
-
Ascorbic acid (vitamin C) does not contain a traditional carboxylic acid group, but it is, nevertheless, still fairly acidic (pKa = 4.2). Identify the acidic proton, and explain your choice using...
-
How can government expenditures be financed?
-
Problem 228: The derivative is dz dt = = atb where a , and b =
-
Write a Python program which will take N names from the user. Create a dictionary from the N names that will hold First_name, Middle_name and Last_name in separate keys. The inputs will take N at...
-
2 Finding Poles and Zeros from a Bode Plot Consider the magnitude portion of the Bode plot in Figure 3. Based on the linear approxi- mation in red, find the transfer function G(s). 5 0 -5 10 -10 -15...
-
Indicate whether the following statements are "TRUE" or "FALSE" 1- Financial accounting is considered to be the backbone to top management. 2- Cost accounting identifies, summarizes and interprets...
-
Refer to case 3 shown above. Assume that Beta Division is now receiving an 3% price discount from the outside supplier. a. What is Alpha Division's lowest acceptable transfer price? b. What is Beta...
-
Solve each system using the substitution method. If a system is inconsistent or has dependent equations, say so. 3x - y = 10 2x + 5y = 1
-
Which internal control principle is especially diffi cult for small organizations to implement? Why?
-
If one were to try to draw the simplest Lewis structure for molecular oxygen, the result might be the following However, it is known from the properties of molecular oxygen and experiments that O2...
-
When ethane is chlorinated, 1,1-dichloroethane and 1,2-dichloroethane, as well as more highly chlorinated ethanes, are formed in the mixture (see Section 10.3A). Write chain reaction mechanisms...
-
(a) What percentages of 1-chloropropane and 2-chloropropane would you expect to obtain from the chlorination of propane if 1( and 2( hydrogen atoms were equally reactive? (b) What percentages of...
-
Berbice Inc. has a new project, and you were recruitment to perform their sensitivity analysis based on the estimates of done by their engineering department (there are no taxes): Pessimistic Most...
-
#3) Seven years ago, Crane Corporation issued 20-year bonds that had a $1,000 face value, paid interest annually, and had a coupon rate of 8 percent. If the market rate of interest is 4.0 percent...
-
I have a portfolio of two stocks. The weights are 60% and 40% respectively, the volatilities are both 20%, while the correlation of returns is 100%. The volatility of my portfolio is A. 4% B. 14.4%...

Study smarter with the SolutionInn App


