Question: In the previous section, we learned how to use malonic ester as a starting material in the preparation of substituted carboxylic acids (the malonic ester
In the previous section, we learned how to use malonic ester as a starting material in the preparation of substituted carboxylic acids (the malonic ester synthesis). That method employed a step in which the enolate of malonic ester attacked an alkyl halide to give an alkylation product. In this section, we saw that the enolate of malonic ester can attack many electrophilic reagents other than simple alkyl halides. Specifically, the enolate of malonic ester can attack any of the Michael acceptors in the following table. Using malonic ester as your starting material and any other reagents of your choice, show how you would prepare each of the following compounds.
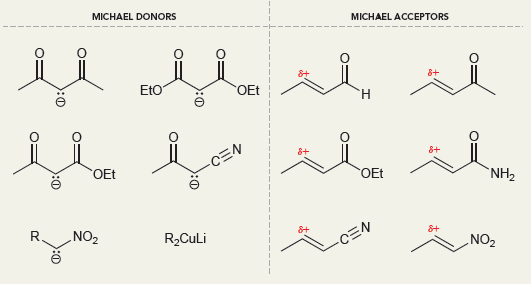
(a)
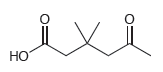
(b)
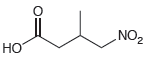
MICHAEL DONORS MICHAEL ACCEPTORS EtO &+ OEt `H &+ CEN OEt OEt `NH2 R. NO2 R2CuLi &+ CEN NO2
Step by Step Solution
3.44 Rating (157 Votes )
There are 3 Steps involved in it
a b Eto ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
1625_606b0df1ca54f_656568.pdf
180 KBs PDF File
1625_606b0df1ca54f_656568.docx
120 KBs Word File


