Predict the position of equilibrium for each of the following reactions: (a) (b) (c) HO NH NH2
Question:
(a)
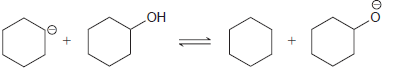
(b)
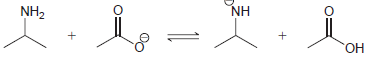
(c)
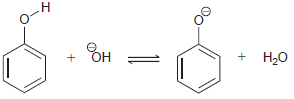
Transcribed Image Text:
HO NH NH2 ОН
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (12 reviews)
a The rig...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Determine the position of equilibrium for each acid-base reaction below: (a) (b) (c) (d) `H. O + H20
-
Draw the products of each of the following acid-base reactions, and then predict the position of equilibrium in each case: (a) (b) NaH
-
In each case below, identify the acid and the base. Then draw the curved arrows showing a proton transfer reaction. Draw the products of that proton transfer, and then predict the position of...
-
Each student will interview a manager or an employee (who might be a family member, a friend, or an acquaintance) to determine the extent to which the issues raised in the case are represented in his...
-
In the short-run (before P adjusts) version of the IS-LM model, a temporary decrease in government purchases causes the real interest rate to (blank) and output to (blank): a. rise; rise b. rise;...
-
Use the laws of exponents to simplify the algebraic expressions. Your answer should not involve parentheses or negative exponents. (16x 8 ) -3/4
-
Observation or experiment? The Nurses Health Study has queried a sample of over 100,000 female registered nurses every two years since 1976. Beginning in 1980, the study asked questions about diet,...
-
Given the following code letters and components of financial statements indicate where each item would most likely be reported in the financial statements by inserting the corresponding code letters....
-
Accrual Accounting Concepts 1. Lansing Cycles failed to record an adjusting entry for accrued expenses at the end of June. What effect does this have on the financial statements? a. Expenses are...
-
Design a database for a world-wide package delivery company (e.g., DHL or FedEX). The database must be able to keep track of customers (who ship items) and customers (who receive items): some...
-
Amphotericin B is a powerful antifungal agent used for intravenous treatment of severe fungal infections. Identify the most acidic proton in this compound: , Amphotericin B NH2
-
As we will learn in Chapter 21, treating a lactone (a cyclic ester) with sodium hydroxide will initially produce an anion: This anion rapidly undergoes an intramolecular proton transfer, in which the...
-
Will you expect a positive, zero, or negative linear correlation between the two variables for each of the following examples? a. SAT scores and GPAs of students b. Stress level and blood pressure of...
-
Write a java program that contain two overloaded methods that accepts two numbers or two characters representing a range example (11, 37) or (c, w) inputted by the user. The method generates a random...
-
Maggie could not conceive a child using natural means, so she sought out a woman who would donate an egg to be surgically implanted in Maggie. Which of the following items are deductible by Maggie in...
-
M corporation is subject to tax only in state b state b law provides for the use of federal taxable income before net operating loss and special deductions as the starting point for computing state...
-
Use Routh Criteria to determine the values of K needed for the system represented by the Characteristic Equation to be stable. (1 + K)s + (2K + 3)s + 2 3K = 0 Obtain the root locus plot for the...
-
Q7 a) Two forces equal to 2P and P act on a particle. If the first be doubled and second is increased by 12N, the direction of resultant remains unaltered. Find the value of P (5)
-
Solve each inequality. 2x + 3 > 5 or x 1 6
-
At the beginning of its fiscal year, Lakeside Inc. leased office space to LTT Corporation under a seven-year operating lease agreement. The contract calls for quarterly rent payments of $25,000 each....
-
Predict the products from each of the following reactions. (a) (b) (c) (d) (e) (f) (g) (h) (i) (j) Hl (excess) Hi H,so,,H20 O MeONa O MeOH, cat. H2SO (1) EtSNa (2) H2o HCl (1 equiv.) MeONa (1)EtON...
-
Provide the reagents necessary to accomplish the following syntheses. (a) (b) (c) (d) MeO MeO SEt SEt
-
Provide reagents that would accomplish the follwing syntheses. (a) (b) HO Glycerol Epichlorohydrin
-
3. The nominal interest rate compounded monthly when your $7,000 becomes $11,700 in eight years is ________
-
An investor can design a risky portfolio based on two stocks, A and B. Stock A has an expected return of 21% and a standard deviation of return of 39%. Stock B has an expected return of 14% and a...
-
Advanced Small Business Certifica Drag and Drop the highlighted items into the correct boxes depending on whether they increase or decrease Alex's stock basis. Note your answers- you'll need them for...

Study smarter with the SolutionInn App


