Predict the product(s) obtained when each of the following compounds is treated with a mixture of nitric
Question:
(a)
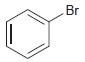
(b)
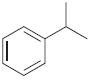
(c)
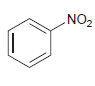
(d)
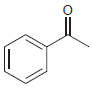
(e)
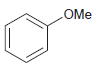
Transcribed Image Text:
Br
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (16 reviews)
a b ...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw the product obtained when each of the following compounds is treated with acetic anhydride in the presence of pyridine: (a) -d-Galactopyranose (b) -d-Glucopyranose (c) -d-Galactopyranose
-
Predict the major product obtained when each of the following compounds undergoes a Claisen condensation. (a) (b) (c) OEt OM
-
Predict the product of the Dieckmann cyclization that occurs when each of the following compounds is treated with sodium ethoxide. (a) (b) (c) OEt LOET Eto OEt
-
The United StatesMexicoCanada Agreement replaced what trade agreement?
-
Explain the characteristics of effective objectives. Why is setting objectives important? (LO 3: Develop a strategic plan for a business using the nine steps in the strategic planning process)
-
In Example 2, change the right side of the second equation from 2 to 3 and then solve the system. Data from Example 2 By substitution, solve the system of equations From the first equation, we have y...
-
Faculty Classroom Hours The dean of a university estimates that the mean number of classroom hours per week for full-time faculty is 11.0. As a member of the student council, you want to test this...
-
A bond issued by Zephyr Balloons currently has a market price equal to $1,080. The bond pays $120 interest annually. a. If you buy the bond and its price does not change during the year, what is the...
-
In which of the following circumstances is testing of controls required? when management has established well-designed controls around the processes associated with the estimate when substantive...
-
The estimated Canadian processed pork demand function (Moschini and Meilke, 1992) is Q = 171 - 20p + 20p b + 3p c + 2Y (see Exercise 1.1), and the supply function is Q = 178 + 40p - 60ph (see...
-
Describe the Theory of Constraints (TOC).
-
What is the drum-buffer-rope (DBR) concept and what does it have to do with the TOC?
-
Nike disclosed the following in its 1998 annual report (in millions): www.nike.com Inventory valuation: Inventories are stated at the lower of cost or market. Cost is determined using the last-in,...
-
Solve for "C" and "E": 1) E cos (15)-C=0 2) -300+ C+E sin (15) = 0
-
Let u=3, b. Compute uv, uv, 2-3 v =
-
Using Complex Numbers show that d cosz=-sinz dz
-
use for loops to solve the following problems 1. Write a complete C++ program that does the following. It asks the user to enter their age (which is assumed to be a positive integer). The program...
-
Profile Vickers hardness test Penetrating body: Square diamond pyramid :Test force F N ... 981 N (HV 5 ... HV 100) 49 :Measured value Diagonals of the square impression d Hardness value: F 0,189 F...
-
In Exercises 137140, determine whether each statement makes sense or does not make sense, and explain your reasoning. I can solve 4 x = 15 by writing the equation in logarithmic form.
-
Provide a few individual examples who revealed what aspects of emotional intelligence?
-
What product is obtained in each case when 3-hexyne is treated in each of the following ways? With H2 over Pd/C and quinoline and the product of that reaction with D2 over Pd/C
-
What product is obtained in each case when 3-hexyne is treated in each of the following ways? With H2 over Pd/C and quinoline and the product of that reaction with D2 over Pd/C
-
(a) Using the pKa values of the hydrocarbons and ammonia, estimate the equilibrium constant for (1) the reaction in Eq. 14.22 and (2) the analogous reaction of an alkane with amide ion. Eq. 14.22 (b)...
-
5. Which of the following is the cheapest for a borrower? a. 6.7% annual money market basis b. 6.7% semi-annual money market basis c. 6.7% annual bond basis d. 6.7% semi-annual bond basis.
-
Waterloo Industries pays 30 percent corporate income taxes, and its after-tax MARR is 24 percent. A project has a before-tax IRR of 26 percent. Should the project be approved? What would your...
-
Imagine you are an Investor in the Stock Market. Identify three companies in the Korean Stock Market (KOSPI) where you would like to invest. Explain your answer

Study smarter with the SolutionInn App


