Predict which of the following compounds is more acidic, and explain your choice. N- -N- -NH2 NH2
Question:
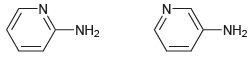
Transcribed Image Text:
N- -N- -NH2 NH2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (12 reviews)
Compare the conjugate bases Both are resonance stabilized ...View the full answer

Answered By

Irfan Ali
I have a first class Accounting and Finance degree from a top university in the World. With 5+ years experience which spans mainly from the not for profit sector, I also have vast experience in preparing a full set of accounts for start-ups and small and medium-sized businesses. My name is Irfan Ali and I am seeking a wide range of opportunities ranging from bookkeeping, tax planning, business analysis, Content Writing, Statistic, Research Writing, financial accounting, and reporting.
4.70+
249+ Reviews
530+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Predict which of the following compounds is more acidic. After making your prediction, use the pK a values from the following table to determine whether your prediction was correct. ACID pka...
-
Identify which of the following compounds is more acidic and explain your choice.
-
Identify which of the following compounds is more acidic. Explain your choice. C=C H--
-
Lewis and Associates has been in the termite inspection and treatment business for five years. The following is a list of accounts for Lewis on June 30, 2017. It reflects the recurring transactions...
-
On a foreign exchange market diagram with the dollar price of the British pound on the vertical axis, explain why you draw an upward-sloping supply curve.
-
To calculate the number of days in the holding period, a taxpayer should: a. Include the date of acquisition b. Exclude the date of disposition c. Exclude the date of acquisition d. Include the date...
-
Charity The table shows the results of a survey that asked 2850 people whether they were involved in any type of charity work. A person is selected at random from the sample. Find the probability of...
-
Heather Hudson makes stuffed teddy bears. Recent information for her business follows: Selling price per bear ....... $35.00 Total fixed cost per month ..... 1,500.00 Variable cost per bear ..........
-
A financial institution has the following market value balance sheet structure: Assets Liabilities and Equity Cash $ 3 , 0 0 0 Certificate of deposit $ 1 1 , o 0 0 Bond 1 0 , 0 0 0 Equity 2 , 0 0 0...
-
Audience Benefits and the You View Your Task. Revise the following sentences to emphasize the audiences perspective and the you view. a. We regret to announce that the bookstore will distribute free...
-
Sigma bonds experience free rotation at room temperature: In contrast, Ï bonds do not experience free rotation. Explain. . cec - `H
-
Consider the reaction below. The rate of this reaction is markedly increased if a small amount of sodium iodide is added to the reaction mixture. The sodium iodide is not consumed by the reaction and...
-
The baggage limit for an airplane is set at 100 pounds per passenger. Thus, for an airplane with 200 passenger seats, there would be a limit of 20,000 pounds. The weight of the baggage of an...
-
The Role of Leadership in Shaping Organizational Culture Recent research stated that [c]ompanies with an established organizational culture that includes strong capabilities for change, commitment to...
-
Unscheduled absences by clerical and production workers are an important cost in many companies. Reducing the rate of absenteeism is, therefore, an important goal for a company's human relations...
-
Many of the largest tech firms, including Google, Apple, Amazon, and Microsoft, have spent hundreds of millions of dollars to improve their information technology infrastructure. Now, these companies...
-
In the business sense, a product refers to a commodity available for purchase, encompassing both services and tangible or intangible items. It may exist in physical, virtual, or cyber forms. Every...
-
Data Exploration and Multiple Linear Regression (MLR) using SAS. The "College" data set contains the statistics for many US Colleges from 1995 issue of US News and World Report. It has 777...
-
Use the fact that a x = a y implies x = y, to solve each equation. 1+2x =
-
The vapor pressure of the liquid NH, is measured at different temperatures. The following vapor pressure data are obtained. Temperature, K P, mmHg 217.1 223.4 234.7 588.1 Calculate the enthalpy of...
-
Epoxide?s react with Grignard reagents to yield alcohols propose a mechanism. 1. CHMgBr 2. * CH
-
How would you prepare the following substances from Cyclopentanol? More than one step may be required. (a) Cyclopentanone (b) Cyclopentane (c) 1-Methylcyclopentanol (d) Trans-2-Methylcyclopentanol I...
-
What products would you expect to obtain from reaction of 1-methylcyclo-hexanol with the following reagents? (a) HBr (b) NaH (c) H2SO4 (d) Na2Cr2O7
-
Flexible manufacturing places new demands on the management accounting information system and how performance is evaluated. In response, a company should a. institute practices that reduce switching...
-
Revenue and expense items and components of other comprehensive income can be reported in the statement of shareholders' equity using: U.S. GAAP. IFRS. Both U.S. GAAP and IFRS. Neither U.S. GAAP nor...
-
Kirk and Spock formed the Enterprise Company in 2010 as equal owners. Kirk contributed land held an investment ($50,000 basis; $100,000 FMV), and Spock contributed $100,000 cash. The land was used in...

Study smarter with the SolutionInn App


