Propose a mechanism that explains formation of the following product: H2SO4 Heat
Question:
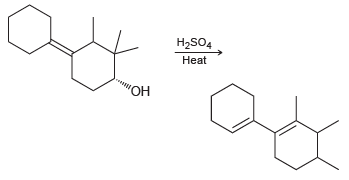
Transcribed Image Text:
H2SO4 Heat "ОН
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 28% (14 reviews)
OH sofor ...View the full answer

Answered By

Fahmin Arakkal
Tutoring and Contributing expert question and answers to teachers and students.
Primarily oversees the Heat and Mass Transfer contents presented on websites and blogs.
Responsible for Creating, Editing, Updating all contents related Chemical Engineering in
latex language
4.40+
8+ Reviews
22+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Write a mechanism that explains formation of the products shown in the following reaction. Br, NaCI, H20o Rr CL
-
Provide a mechanism that explains formation of the following products. Include all intermediates, formal charges, and arrows showing electron flow. 0 N-Br (NBS) 0 hv 11
-
Provide a mechanism that explains formation of the following products. CI HCI (concdCI+
-
Access various employment Web sites (for example, www.monster.com and www.dice.com ) and find several job descriptions for a database administrator. Are the job descriptions similar? What are the...
-
Write a paper, response to the following question. Do not use the question in your response. Read the case study on Undercover Advertising "Viral Marketing." After reading the case study, take a...
-
these are the answer sheet please fill this XY Consumer Products GENERAL JOURNAL GJ01 Date General Ledger 2003 Description Acc # Debit Credit Mar. 31 Bank Charges & Interest Mar. 31 Employers Payroll...
-
Why is productive maintenance important? LO,1
-
If a company wants to implement an enterprise application, it had better do its homework. Discuss the implications of this statement.
-
Required information [The following information applies to the questions displayed below. Matt and Meg Comer are married and file a joint tax return. They do not have any children. Matt works as a...
-
Peyton Company reported these ratios at December 31, 2018 (dollar amounts in millions): Peyton Company completed these transactions during 2019: a. Purchased equipment on account, $5 b. Paid...
-
Draw all constitutional isomers of C 4 H 9 Br, and then arrange them in order of increasing reactivity toward an E2 reaction.
-
(S)-1-Bromo-1,2-diphenylethane reacts with a strong base to produce cis-stilbene and trans-stilbene: a) This reaction is stereo-selective, and the major product is trans-stilbene. Explain why the...
-
In what way does the presence of junk-mail advertising in e-mail and pop-up ads on websites create conflict? Who are the parties in conflict?
-
The COVID pandemic has created a crisis for many restaurateurs. The author of one of this week's readings has a suggestion that he thinks could help restaurants survive the crisis. Read the article...
-
Evidence is used to make a decision whenever the decision follows directly from the evidence (Tingling & Brydon, 2010). This is where so many people get it wrong or going by their personal beliefs or...
-
Pick 2 countries, find the price of a Big Mac in each country (if you want to pick another good/service, go ahead), express the price in the local currency, then with the help of exchange rate,...
-
Your task is to educate the public about the role of the Fed in the economy. Role: You are an economic issues reporter for PBS. Audience: Television audience of The Newshour on PBS Situation: Your...
-
Trade Queens Limited is a highly successful FMCG in Zambia. Salient points from the Year-end report indicate the following: Operating profit for the 2022 financial year is up 60% year on year,...
-
In Exercises 17 through 32, sketch the graph of the given function. f(x) = 1 Vx X 1 -
-
The population of Detroit, Michigan, decreased from 1,027,974 in 1990 to 688,701 in 2013 (Source: U.S. Census Bureau). Find the average rate of change in the population of Detroit, Michigan, over the...
-
Predict the number of carbon resonance lines you would expect in the 13C NMR spectra of the following compounds: (a) Methylcyclopentane (b) 1-Methylcyclohexene (c) 1, 2-Dimethylbenzene (d)...
-
Propose structures for compounds that fit the following descriptions: (a) A hydrocarbon with seven lines in its 13C NMR spectrum (b) A six-carbon compound with only five lines in its 13C NMR spectrum...
-
Assign the resonances in the 13C NMR spectrum of methyl propanoate, CH3CH2CO2CH3(figure). TMS CH-cH 2 1 120 200 180 160 140 100 20 40 O ppm 60 Chemical shift (8) Intensity
-
Saly paid $52,000 a year paid on a weekly basis. last pay she had $250 withheld in Income Tax, $48.97 for CPP and $15.80 for EI. an additional $and 25.00 in tax are deducted each pay. She allowed to...
-
Required information [The following information applies to the questions displayed below.] Dain's Diamond Bit Drilling purchased the following assets this year. Asset Drill bits (5-year) Drill bits...
-
Which of the following partnership items are not included in the self-employment income calculation? Ordinary income. Section 179 expense. Guaranteed payments. Gain on the sale of partnership...

Study smarter with the SolutionInn App


