(S)-1-Bromo-1,2-diphenylethane reacts with a strong base to produce cis-stilbene and trans-stilbene: a) This reaction is stereo-selective, and...
Question:
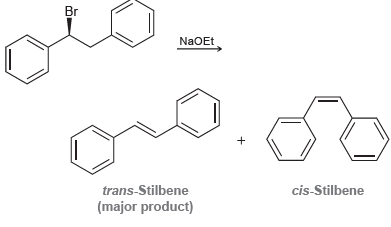
a) This reaction is stereo-selective, and the major product is trans-stilbene. Explain why the trans-isomer is the predominant product. To do so, draw the Newman projections that lead to formation of each product and compare their stability.
b) When (R)-1-bromo-1,2-diphenylethane is used as the starting substrate, the stereo-chemical outcome does not change. That is, trans-stilbene is still the major product. Explain.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





