Starting with 1,3-butadiene as your only source of carbon atoms, and using any other reagents of your
Question:
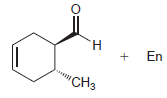
Transcribed Image Text:
н En "CHз
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (10 reviews)
HBr 40 ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Using acetylene as your only source of carbon atoms, design a synthesis of pen-tanal. (Pentanal has an odd number of carbon atoms, while acetylene has an even number of carbon atoms): H.
-
Using acetylene as your only source of carbon atoms, identify a synthetic route for the production of 2-bromobutane.
-
Using acetylene as your only source of carbon atoms, identify a synthetic route for the production of 1-bromobutane.
-
Why " Kodak " is unsuccessful in implementing a strategy. Can you prepare a critical examination of the strategy to address the following questions about Kodak. What was the strategy and why do you...
-
For the element shown in the figure: (a) Find the magnitude of the unknown stresses h and h on the horizontal plane. (b) Find the orientation of the principal stresses; clearly indicate their...
-
A point is moving along the curve in such a way that the x-coordinate is increasing at a constant rate of 0.02 units per second. Find the rate at which the y-coordinate is changing when x = 2,...
-
Every touch point is considered, explains Stephanie Perdue, Taco Bells senior director of marketing, from the posters in the restaurants down to the packaging, and all of the different media...
-
Sunco processes oil into aviation fuel and heating oil. It costs $40 to purchase each 1000 barrels of oil, which is then distilled and yields 500 barrels of aviation fuel and 500 barrels of heating...
-
Camaro $ 2,650 0 310 2,300 400 $ 5,660 $ 2,320 Cash Short-term investments Current receivables Inventory Prepaid expenses Total current assets Current liabilities GTO $ 280 0 560 2,120 700 $3,660...
-
A temperature scale that never quite caught on was formulated by the Austrian chemist Johann Sebastian Farblunget. The reference points on this scale were 0FB, the temperature below which Farblungets...
-
Expert Analysts Resources (EAR) has provided you with the following information about three companies you are currently evaluating: According to this information, which firm would be considered...
-
People drive faster when they have auto insurance. This is an example of: a. Adverse selection. b. Asymmetric information. c. Moral hazard.
-
The element europium exists in nature as two isotopes: 151 Eu has a mass of 150.9196 amu, and 153 Eu has a mass of 152.9209 amu. The average atomic mass of europium is 151.96 amu. Calculate the...
-
Identify a weakness of your own that might affect your ability to lead change effectively.
-
What makes an ability (or set of abilities) a core competency? Pick a company you are familiar with (I've picked Apple Company) . Can you identify some of its core competencies What methods do you...
-
What are the key standards and frameworks commonly used by IS auditors during the IS audit process, and how do these standards contribute to the effectiveness and reliability of IS audit activities?...
-
What is the definition of a project risk? What is risk threat and Risk Opportunity? What are the responsibilities of the risk or opportunity owner? What alternatives are there to managing "excessive"...
-
6.1. Determine the transfer function H(s)/Q(s) for the liquid-level system shown in Fig. P61. Resistances R1 and R2 are linear. The flow rate from tank 3 is maintained constant at b by means of a...
-
Problems 2837 are based on material learned earlier in the course. The purpose of these problems is to keep the material fresh in your mind so that you are better prepared for the final exam. If the...
-
Complete the equations for the following equilibria and calculate Keq where the Keq expression includes [HO]. Be sure to enter Keq in proper scientific notation. (a) ammonia (acting as a base) reacts...
-
If methanol rather than water is added at the end of a Hell-Volhard-Zelinskii reaction, an ester rather than an acid is produced. Show how you could carry out the following transformation, and...
-
Identify the most acidic hydrogens in each of the following molecules: (a) CH3CH2CHO (b) (CH3) CH3CCOCH3 (c) CH3CO2H (d) Benz amide (e) CH3CH2CH2CN (f) CH3CON (CH3)2
-
Draw a resonance structure of the acetonitrile anion, :CH 2 C N, and account for the acidity of nitriles.
-
When preparing government-wide financial statements, the modified accrual based governments funds are adjusted. Please show the adjustments (in journal entry form with debits and credits) that would...
-
I need help finding the callable price and call value
-
On 31 October 2022, the owner took goods for his son as a birthday gift. The cost price of the goods was R15 000

Study smarter with the SolutionInn App


