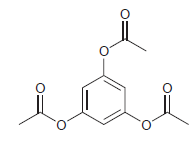
The following compound is highly activated, but nevertheless undergoes bromination very slowly. Explain.
Question:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (7 reviews)
All three ...View the full answer

Answered By

Anum Naz
Lecturer and researcher with 10+ years of experience teaching courses in both undergraduate and postgraduate levels. Supervised 17 BA theses, 07 MA theses, and 1 Ph.D. dissertations. Edited and co-authored 2 monographs on contemporary trends in political thought. Published over articles in peer-reviewed journals.
4.80+
11+ Reviews
52+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following compound is optically inactive. Explain why.
-
Which nitrogen atom in the following compound is more basic?Explain.
-
The following compound is not aromatic even though it has 4n + 2 electrons in a continuous cyclic array. Explain why this compound is not aromatic.
-
Explicitly justify relationships (11.5.3) between the compliances of the plane stress and plane strain theories. Equation 11.5.3 B11 B22 B66 = S11 S33-S2 $33 S22 S33-S23 S33 S66 S33 - S36 S33 B12 B16...
-
Why is it important for entrepreneurs to define their target markets as part of their marketing strategies?
-
The Alpine House, Inc. is a large winter sports equipment broker. Below is an income statement for the company's ski department for a recent quarter. LA CASA ALPINA, INC. Income Statement - Ski...
-
E 20-11 General fund journal entries Prepare entries in the general fund to record the following transactions and events:
-
Construct a Gantt chart for the following set of activities and indicate the project completion time and slack for eachactivity: Activity Activity Predecessor Time (weeks) 6
-
Candice Willis will invest $34,700 today. She needs $101,920 in 14 years. Click here to view factor tables What annual interest rate must she earn? (Round answer to 0 decimal places, e.g. 7%.)...
-
Recently, Ashland MultiComm Services has been criticized for its inadequate customer service in responding to questions and problems about its telephone, cable television, and Internet services....
-
For the reaction C(graphite) + H 2 O(g) CO(g) + H 2 (g), H o R =131.28 kJ mol -1 at 298.15 K. Use the values of C P,m at 298.15 K in the data tables to calculate H R at 125.0C.
-
Ca(HCO 3 ) 2 (s) decomposes at elevated temperatures according to the stoichiometric equation Ca(HCO 3 ) 2 (s) ???? CaCO 3 (s) + H 2 O(g) + CO 2 (g). a. If pure Ca(HCO 3 ) 2 (s) is put into a sealed...
-
What attributes must accounting information possess to be reliable?
-
Determine the magnitude of the magnetic flux through the south-facing window of a house in British Columbia, where Earth's B field has a magnitude of 5.8 x 10-5T and the direction of B field is 72...
-
A wedge with an inclination of angle rests next to a wall. A block of mass m is sliding down the plane, as shown. There is no friction between the wedge and the block or between the wedge and the...
-
Conner Leonard worked for Purges Manufacturing for 32 years. Along with four other men, he helped to start the company that designed and built products sold around the world. Purges Manufacturing...
-
Reconsider the collision between two objects diagrammed below where two objects move on a frictionless surface. Before collision After collision Experiment 1 A, 1 B A B Draw complete and properly...
-
3. Now the bomb arrives. Please catch fx,y(x, y) = = cx cx - dy, where 0 < x < 1, 0 y x. 13 a) Please find coefficients c, d such that cd= 8 b) Please find fx(x) and fy (y). Are X and Y independent?...
-
In Exercises 130133, write the equation of a rational function f(x) = p(x)/q(x) having the indicated properties, in which the degrees of p and q are as small as possible. More than one correct...
-
Prepare a stock card using the following information A company is registered for GST which it pays quarterly, assume GST was last paid on the 30th of June 2019. It uses weighted average cost...
-
Rationalize the indicated fragments in the EI mass spectrum of each of the following molecules by proposing a structure of the fragment and a mechanism by which it is produced. (a)...
-
A chemist, Ilov Boronin, carried out a reaction of frans-2-pentene with BH3 in THF followed by treatment with H2O2/-OH. Two products were separated and isolated. Desperate to know their structures,...
-
Rationalize each of the following observations by postulating a structure for the fragment ion(s) and the mechanisms for their formation. The EI mass spectrum of 2-methoxybutane shows a base peak at...
-
you are analyzing the cost of debt for a firm. Do you know that the firms 14 year maturity, 7.8 Percent coupon bonds are selling at a price of $834. The Barnes pay interest semi annually. If these...
-
***Please answer the following using excel and showcasing the formulas/calculations used*** thank you so much Financial information on AAA Ltd. is shown below. AAA Ltd. Income Statement For the Year...
-
2. In an account Anh Paglinawan currently has $216,670.00. At a rate of 8.00% how long will it take for them to have $298,390.00 assuming semi-annually compounding? (Hint: compute the exact years, do...

Study smarter with the SolutionInn App


