We saw a general rule that the two protons of a CH 2 group will be chemically
Question:
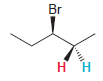
Transcribed Image Text:
Br нн Н
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (10 reviews)
The presence of the bromine atom does not render C 3 a chirality c...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
It is a general rule that any organic compound containing oxygen, nitrogen, or a multiple bond will dissolve in concentrated sulfuric acid. Explain the basis of this rule in terms of acid-base...
-
Two protons in an atomic nucleus repel each other, but they are also attracted to each other. Explain.
-
Two protons approach each other with different speeds. (a) Will the magnitude of the total momentum of the two-proton system be (1) greater than the magnitude of the momentum of either proton, (2)...
-
Consider Devine Fashion from S8-6. Assume that the fixed expenses assigned to each department include only direct fixed costs of the department (rather than unavoidable fixed costs as given in S8-6):...
-
What factors cause the differences in yields on U.S. government bonds?
-
How do bailments for value differ from gratuitous bailments?
-
Choose the statement which you consider to be correct out of the following: (i) A Bank Reconciliation Statement is prepared so that (a) The difference in the balance in the bank and the cash balances...
-
Digital Software Inc., has two product lines. The income statement for the year ended December 31 shows the following: The products, Num 1 and Num 2, are sold in two territories, North and South, as...
-
Standard (New) Block Advisors Small Business Certification Test (2021) Tax in Small Business: Record Reconstruction Question 18 of 35. A sole proprietor may calculate their business gross income...
-
There is a lottery with n coupons and n people take part in it. Each person picks exactly one coupon. Coupons are numbered consecutively from 1 to n, n being the maximum ticket number. The winner of...
-
Identify the number of signals expected in the 1 H NMR spectrum of each of the following compounds. (a) (b) (c) (d) (e) (f) (g) (h) (i) (j) (k) (l) OMe MeO.
-
Identify the structure of a compound with molecular formula C 9 H 20 that exhibits four CH 2 groups, all of which are chemically equivalent. How many total signals would you expect in the 1 H NMR...
-
Using (7), find the potential (r, ) in the unit disk r < 1 having the given boundary values (1, ). Using the sum of the first few terms of the series, compute some values of and sketch a figure of...
-
Jason is a sole trader in the architecture industry. For the year ending 30 June 2019, Jason hired a 3D model designer, Sarah, to help him with the growing business. At the end of the year he has the...
-
Read Case 14-1 Trojan Technologies (15th ed., p. 426 OR 16th ed., p. 431) Guiding Questions and additional information: In preparing your case study, ensure that you answer the following questions:...
-
Jorge Rimert works for Road to Success Collection Agency. He oversees mailing out collection notices to patients. Upon review of the patients who have not paid from Hideaway Hospital, Jorge notices...
-
An epidemiologist plans to conduct a survey to estimate the percentage of women who give birth. How many women must be surveyed in order to be 99% confident that the estimated percentage is in error...
-
A 100 acre plot of land has a concentration time of 80 minutes. The area is residential single family homes with a C-0.40. What is the percent Increase in stormwater runoff from a 50 year recurrence...
-
Approximate f(x) to four decimal places. f(x) = 4(e-0.3x = -0.6x), - x = 1.6
-
Before the 1973 oil embargo and subsequent increases in the price of crude oil, gasoline usage in the United States had grown at a seasonally adjusted rate of 0.57 percent per month, with a standard...
-
Draw the structures of the following molecules: (a) Biacetyl, C4H6O2, a substance with the aroma of butter; it contains no rings or carboncarbon multiple bonds. (b) Ethylenimine, C2H5N, a substance...
-
Draw structures for the following: (a) 2-Methyiheptane (b) 4-Ethyl-2, 2-dimethylhexane (c) 4-Ethyl-3, 4-dimethyloctane (d) 2, 4, 4-Trimethylheptane (e) 3, 3-Diethyl-2, 5-dimethylnonane (f)...
-
Draw a compound that: (a) Has only primary and tertiary carbons (b) Has no secondary or tertiary carbons (c) Has four secondary carbons
-
When credit terms for a sale are 2/15, n/40, the customer saves by paying early. What percent (rounded) would this savings amount to on an annual basis
-
An industrial robot that is depreciated by the MACRS method has B = $60,000 and a 5-year depreciable life. If the depreciation charge in year 3 is $8,640, the salvage value that was used in the...
-
What determines a firm's beta? Should firm management make changes to its beta? Be sure to consider the implications for the firm's investors using CAPM.

Study smarter with the SolutionInn App


