When benzene is treated with D 2 SO 4 , a deuterium atom replaces one of the
Question:
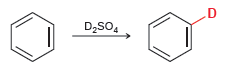
Transcribed Image Text:
D2SO,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (10 reviews)
H b8...View the full answer

Answered By

Ankit Mahajan
I am an electrical engineering graduate from Thapar institute of engineering and technology.
Qualified exams - GATE 2019,2020.
CAT EXAM 2021- 91.4 percentile
SSC EXAMS- 2019,2020,2021
AFCAT EXAM- 2019,2020,2021
I want to share my knowledge with other people so that they can achieve the same.
I have strong hold Mathematics, Electrical engineering and all the subjects related.
Just give me a problem and I will give you the solution of it.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
When benzene is treated with D2SO4, deuterium slowly replaces all six hydrogens in the aromatic ring. Explain.
-
When benzene is treated with propene and sulfuric acid (see structures of reactants below), two different monoalkylation products are possible. Draw their structures. Which one do you expect to be...
-
When benzene is treated with excess D2SO4 at room temperature, the hydrogens on the benzene ring are gradually replaced by deuterium. Write a mechanism that explains this observation.
-
Migration is a popular strategy among many species. Monarch butterflies migrate between the Sierra Madre mountains in Mexico and many locations across the USA and Canada. Answer the following...
-
Should a small business owner always purchase the products with the lowest prices? Why or why not?
-
Determine the number of real solutions of the given systems of equations by sketching the indicated curves. y = 4ln x xy = 6
-
Deceiving subjects. Students sign up to be subjects in a psychology experiment. When they arrive, they are told that interviews are running late and are taken to a waiting room. The experimenters...
-
Tri-State Bank is investigating the processing time for loan applications. Samples were taken for 25 random days from 4 branches. These data can be found in the worksheet C16P5 in the OM5 Data...
-
Question 3 ( 1 point ) Which of the following is NOT a function of a product's packaging? displaying the actual product. revealing the benefit of the product. presenting required information....
-
A stepped shaft ABC consisting of two solid circular segments is subjected to torques T1 and T2 acting in opposite directions, as shown in the figure. The larger segment of the shaft has diameter d1...
-
Calculate A o R and G o R for the reaction C 6 H 6 (l) + 15/2O 2 (g) 6CO 2 (g) + 3H 2 O(l) at 298 K from the combustion enthalpy of benzene and the entropies of the reactants and products.
-
A specialty fabrication shop has the following assembly processes for one of the products it makes: Process 1 feeds a completed component to Process 2; Process 2 feeds a completed assembly to Process...
-
You own a portfolio consisting of the stocks below: The risk- free rate is 3 percent. Also, the expected return on the market portfolio is 11 percent. a. Calculate the expected return of your...
-
A liquid mixture of 65 mole% n-nonane and 35 mol% n-octane enters a flash unit. In the flash unit, the pressure is reduced to 1 atm and half of the liquid is evaporated. find the temperature in the...
-
To gain a deep understanding of SAPPI LIMITED's industry and competitive environment, answer the following questions before the company can embark on a "new strategy" breakaway. Does this industry...
-
What communication tools can a manager use to construct and deliver constructive and timely feedback to their employees? Discuss the various communication tools (i.e. email, phone, text, social...
-
The production per hour, output per unit time, and the number of operations per hour are all examples of labor standards (David & Davis, 2020). Employees will experience both good and negative...
-
Explain in detail on the following CLO 5 -Evaluate strategic implementation and control principles/improvement strategies for business control, including use of strategic Dashboards and Balance...
-
In Exercises 4748, find an nth-degree polynomial function with real coefficients satisfying the given conditions. If you are using a graphing utility, graph the function and verify the real zeros and...
-
Interest Compounded Annually. When P dollars is invested at interest rate i, compounded annually, for t years, the investment grows to A dollars, where A = P(1 + i) t . Trevor's parents deposit $7800...
-
(a) Two isomeric S"2 products are possible when sodium thiosulfate is allowed to react with one equivalent of methyl iodide in methanol solution. Give the structures of the two oroducts. ...
-
When methyl iodide at 0.I M concentration is allowed to react with sodium ethoxide at 0.1 M concentration in ethanol solution, the product ethyl methyl ether is obtained in good yield. Explain why...
-
When methyl iodide at 0.I M concentration is allowed to react with sodium ethoxide at 0.1 M concentration in ethanol solution, the product ethyl methyl ether is obtained in good yield. Explain why...
-
3. The nominal interest rate compounded monthly when your $7,000 becomes $11,700 in eight years is ________
-
An investor can design a risky portfolio based on two stocks, A and B. Stock A has an expected return of 21% and a standard deviation of return of 39%. Stock B has an expected return of 14% and a...
-
Advanced Small Business Certifica Drag and Drop the highlighted items into the correct boxes depending on whether they increase or decrease Alex's stock basis. Note your answers- you'll need them for...

Study smarter with the SolutionInn App


