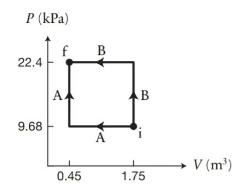
Figure P20.38 shows two processes, A and B, for moving (3.45 times 10^{22}) particles of a monatomic
Question:
Figure P20.38 shows two processes, A and B, for moving \(3.45 \times 10^{22}\) particles of a monatomic ideal gas from state \(\mathrm{i}\) to state \(\mathrm{f}\).
(a) Which process requires less work done on the gas?
(b) Which process requires a smaller quantity \(Q\) of energy transferred thermally to the gas?
(c) By how much does the work done on the gas in process A differ from the work done on the gas in process \(\mathrm{B}\) ?
Data from Figure P20.38
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





