A pair of gas-phase reactions with the following stoichiometric equations take place in a continuous reactor: The
Question:
A pair of gas-phase reactions with the following stoichiometric equations take place in a continuous reactor:
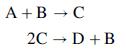
The reactions of ethylene and water to form ethanol and of ethanol to form diethyl ether and water constitute such a reaction system.
(a) Suppose the reactor feed contains A, B, and inerts (I), with mole fractions xA0, xB0, and xI0, respectively. Letting fA denote the fractional conversion of A (mol A consumed/mol A fed) and YC the yield of C based on consumption of A (mol C generated/mol A consumed), prove that for a basis of 1 mol of feed, the number of moles of each species at the outlet are as follows:
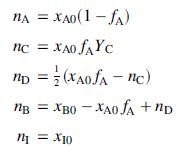
(b) Write a spreadsheet to perform material and energy balance calculations for a basis of 1.00 mol feed. The program should take as inputs
(i) the standard heats of formation (kJ/mol) of A(g), B(g), C(g), and D(g);
(ii) the coefficients (a, b, c, d) of the formulas Cp = a + bT + cT2 + dT3 for gaseous A, B, C, D, and I, where Cp has units of kJ/(mol °C);
(iii) the feed and product temperatures, Tf(°C) and Tp(°C);
(iv) xA0, xB0, fA, and YC.
It should generate an inlet–outlet enthalpy table based on elemental species at 25°C as references and then calculate the required heat transfer to or from the reactor, Q(kJ). The spreadsheet should be tested using the species and reactions of Problem 9.24 and should appear as shown below. Some of the input data and calculated results are shown.
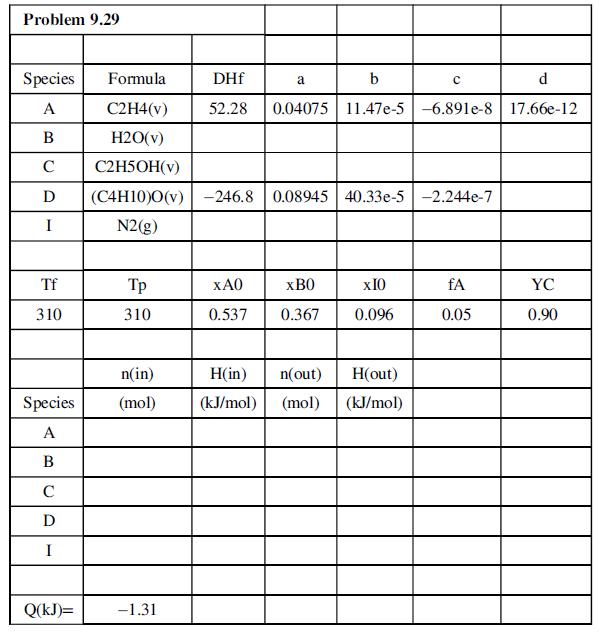
where DHf [= ΔĤ°f(kJ/mol)] denotes the standard heat of formation.
(c) Use the program to calculate Q at the reactor conditions shown in the spreadsheet, then for a feed temperature of 175°C and all other input parameters the same. (The enthalpy table and the value of Q should automatically correct themselves as soon as you type in the new value of Tf.) Print out and turn in your program output for the second feed temperature.
(d) Run the program for several different values of Tp, fA, and YC. Summarize the effects of each of these parameters on Q and briefly explain why your results make sense.
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





