Aqueous sulfuric acid solutions containing 15.0 wt% H 2 SO 4 and 80.0 wt% H 2 SO
Question:
Aqueous sulfuric acid solutions containing 15.0 wt% H2SO4 and 80.0 wt% H2SO4 are mixed to form a 30.0 wt% product solution. The 15% solution was in a laboratory in which the temperature was 77°F. The 80% solution had just been taken from a storage cabinet in an air conditioned stockroom and was at a temperature of 60°F when the mixing occurred.
(a) The mass of the 15% solution is 2.30 lbm. What mass of 80% solution should be weighed out?
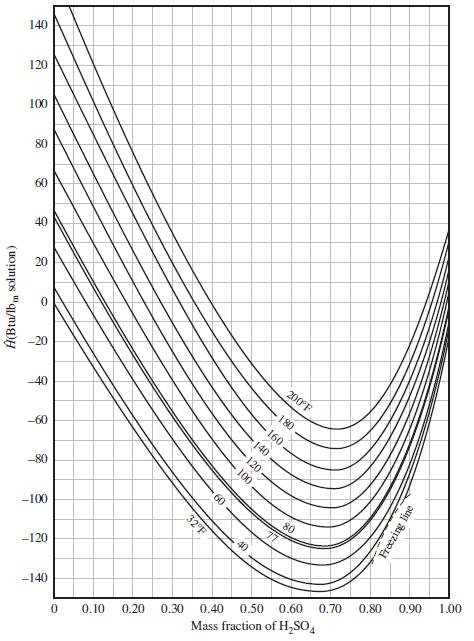
(b) Use Figure 8.5-1 to estimate the product solution temperature if the mixing is adiabatic.
(c) The product solution temperature eventually drops to (77°F). How much heat (Btu) is transferred from the solution to the laboratory air in this constant-pressure cooling process?
(d) The dilution can be carried out either by slowly adding the 15% solution to the 80% solution or vice versa. Use Figure 8.5-1 to determine the maximum temperature that would be attained using each procedure. Which procedure would be safer?
Figure 8.5-1
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





