Question: Benzyne has long been implicated as an intermediate in nucleophilic aromatic substitution; for example, Although the geometry of benzyne has yet to be conclusively established,
Benzyne has long been implicated as an intermediate in nucleophilic aromatic substitution; for example,
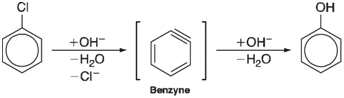
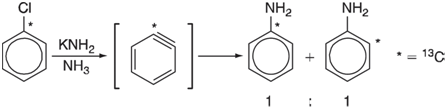
Although the geometry of benzyne has yet to be conclusively established, the results of a 13C labeling experiment leave little doubt that two (adjacent) positions on the ring are equivalent
There is a report, albeit controversial, that benzyne has been trapped in a low-temperature matrix and its infrared spectrum recorded. Furthermore, a line in the spectrum at 2085 cmˆ’1 has been assigned to the stretching mode of the incorporated triple bond. Optimize the geometry of benzyne using the HF/6-31G* model and calculate vibrational frequencies. For reference, perform the same calculations on 2-butyne. Locate the C‰¡C stretching frequency in 2-butyne and determine an appropriate scaling factor to bring it into agreement with the corresponding experimental frequency (2240 cmˆ’1). Then, identify the vibration corresponding to the triple-bond stretch in benzyne and apply the same scaling factor to this frequency. Finally, plot the calculated infrared spectra of both benzyne and 2-butyne. Does your calculated geometry for benzyne incorporate a fully formed triple bond? Compare with the bond in 2-butyne as a standard. Locate the vibrational motion in benzyne corresponding to triple bond stretch. Is the corresponding (scaled) frequency significantly different (>100 cmˆ’1) from the frequency assigned in the experimental investigation? If it is, are you able to locate any frequencies from your calculation that would fit with the assignment of a benzyne mode at 2085 cmˆ’1? Elaborate. Does your infrared plot provide further evidence for or against the experimental observation?
CI 10 +- +OH +- - - . -CI- Benzyne CI NH2 NH2 13C KNH2 NH3
Step by Step Solution
3.34 Rating (166 Votes )
There are 3 Steps involved in it
Benzyne 2butyne benzene According to HF631G calculations the CC stretching frequency in 2butyne is 2... View full answer

Get step-by-step solutions from verified subject matter experts


