Between 0°C and 100°C, the heat capacity of Hg(l) is given by Calculate ÎH and ÎS if
Question:
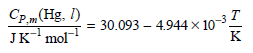
Calculate ΔH and ΔS if 2.25 moles of Hg(l) is raised in temperature from 0.00° to 88.0°C at constant P.
Transcribed Image Text:
Cp.„(Hg. I) 30.093 – 4.944 × 10-32 JK' mol K
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (15 reviews)
36115 AH n Cpmd TK 27315 225 mol 30093 ...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The standard entropy of Pb(s) at 298.15 K is 64.80 J K -1 mol - 1 . Assume that the heat capacity of Pb(s) is given by The melting point is 327.4C and the heat of fusion under these conditions is...
-
The heat capacity of α -quartz is given by The coefficient of thermal expansion is given by β = 0.3530 à 10 4 K 1 and V m = 22.6 cm 3 mol+. Calculate ÎS m for...
-
A 1.75 mole sample of an ideal gas is compressed isothermally from 62.0 L to 19.0 L using a constant external pressure of 2.80 atm. Calculate q, w, U, and H.
-
A 200 g mass attached to a horizontal spring oscillates at a frequency of 1.5 Hz. At one instant, the mass is at x = 70 mm and has vx = -0.2 m/s. Determine (a) The period (b) The amplitude (c) The...
-
Silva Piping Company produces PVC piping in two processing departments-Fabrication and Packaging. Transactions for the month of July are shown as follows. 1. Direct materials totaling $15,000 are...
-
Find a unit normal vector to the surface at the given point. Surface x + y + z = 6 Point (1, 1, 2)
-
6. Consider a large banking group with businesses in retail banking, equity trading, and mergers and acquisitions (M&A) advisory. Discuss its potential for creating value based on the possible...
-
A major issue faced by people who are starting their own business is the form of organization they should select. What are some major characteristics of a partnership that might influence their...
-
Wiater Company operates a small manufacturing facility. On January 1 , 2 0 2 1 , an asset account for the company showed the following balances: During the first week of January 2 0 2 1 , the...
-
The TCM Petroleum Corporation is an integrated oil company headquartered in Fort Worth, Texas. Historical income statements for 2014 and 2015 are found below (dollar figures are in the millions): In...
-
The initiation step for radical addition of HBr is highly endothermic: (a) Explain how this step can be thermodynamically favorable at high temperature even though it is endothermic. (b) Explain why...
-
Draw all resonance structures for each of the following radicals: (a) (b) (c) (d) (e)
-
Why are stakeholder financial products so called?
-
Identify a public conflict (such as a recent Congressional debate or even a celebrity breakup) that has come to the forefront in the media (or public's attention) in the last thirty days. You have...
-
Performance Management Issues You have been asked to return to your alma mater and speak to current students about performance management issues. To make the most of this experience for yourself and...
-
Analysis of competitor organization of our selected organization Walmart and its competitor Safeway. 1. Complete analysis of competitor organization; addresses all relevant factors and typically uses...
-
Defining Program Objectives of Youth centers Clearly define the objectives of your program or center. What specific outcomes do you hope to achieve? Examples may include promoting physical fitness,...
-
Identify a local or regional organization and analyze how they demonstrate servant leadership in their operations. You will want to review their website, social media, news, and other resources to...
-
Let (x) = x 2 - 9, g(x) = 2x, and h(x) = x - 3. Find each of the following. (4) h (x)
-
What is your assessment of the negotiations process, given what you have studied? What are your recommendations for Mr. Reed? You must justify your conclusions
-
Repeat the calculation in Problem 11.4but plot the probability densities of the two orbitals. Then form the difference density, the difference between 2 and | 2a + 2b|
-
Imagine a small electron-sensitive probe of volume 1.00 pm3 inserted into an H+2 molecule-ion in its ground state. Calculate the probability that it will register the presence of an electron at the...
-
The same data as in Problem 11.8 may be used to calculate the molecular potential energy curve for the antibonding orbital, which is given by Plot the curve
-
This question is from case # 24 of book Gapenski's Cases in Healthcare Finance, Sixth Edition Select five financial and five operating Key Performance Indicators (KPIs) to be presented at future...
-
assume that we have only two following risk assets (stock 1&2) in the market. stock 1 - E(r) = 20%, std 20% stock 2- E(r) = 10%, std 20% the correlation coefficient between stock 1 and 2 is 0. and...
-
Flexible manufacturing places new demands on the management accounting information system and how performance is evaluated. In response, a company should a. institute practices that reduce switching...

Study smarter with the SolutionInn App


