The heat capacity of α -quartz is given by The coefficient of thermal expansion is given by
Question:
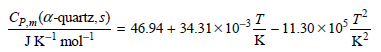
The coefficient of thermal expansion is given by β = 0.3530 × 10ˆ’4 Kˆ’1 and Vm = 22.6 cm3 mol+. Calculate ΔSm for the transformation α -quartz (15.0°C,1atm) †’α -quartz (420.°C, 925 atm).
Transcribed Image Text:
Cp m (a-quartz, s) Jк'mol- 46.94 + 34.31x10-3 - 11.30 x10°, к? к
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (8 reviews)
ASm I Cpm VBPs P dT T 69315 28815 46943431101 113x105 10 1 ...View the full answer

Answered By

Saikumar Ramagiri
Financial accounting:- Journal and ledgers, preparation of trail balance and adjusted trail balance Preparation of income statement, retained earning statement and balance sheet Banks reconciliation statements Financial statement analysis Cash flow statement analysis (both direct and indirect methods) All methods of Depreciations Management Accounting:- Ratios Budgeting control Cash budget and production budget Working capital management Receivable management Costing:- Standard and variance costing Marginal costing and decision making Cost-volume-profit analysis Inventory management (LIFO, FIFO) Preparation and estimation of cost sheet Portfolio management:- Calculation of portfolio standard deviation or risk Calculation of portfolio expected returns CAPM, Beta Financial management:- Time value of money Capital budgeting Cost of capital Leverage analysis and capital structure policies Dividend policy Bond value calculations like YTM, current yield etc International finance:- Derivatives Futures and options Swaps and forwards Business problems Finance problems Education (mention all your degrees, year awarded, Institute/University, field(s) of major): Education Qualification Board/Institution/ University Month/Year of Passing % Secured OPTIONALS/ Major ICWAI(inter) ICWAI inter Pursuing Pursuing - M.com(Finance) Osmania University June 2007 65 Finance & Taxation M B A (Finance) Osmania University Dec 2004 66 Finance & Marketing. B.Com Osmania University June 2002 72 Income Tax, Cost & Mgt, Accountancy, Auditing. Intermediate (XII) Board of Intermediate May 1999 58 Mathematics, Accountancy, Economics. S S C (X) S S C Board. May 1997 74 Mathematics, Social Studies, Science. Tutoring experience: • 10 year experience in online trouble shooting problems related to finance/accountancy. • Since 6 Years working with solution inn as a tutor, I have solved thousands of questions, quick and accuracy Skills (optional): Technical Exposure: MS Office, SQL, Tally, Wings, Focus, Programming with C Financial : Portfolio/Financial Management, Ratio Analysis, Capital Budgeting Stock Valuation & Dividend Policy, Bond Valuations Individual Skills : Proactive Nature, Self Motivative, Clear thought process, Quick problem solving skills, flexible to complex situations. Achievements : 1. I have received an Award certificate from Local Area MLA for the cause of getting 100% marks in Accountancy during my Graduation. 2. I have received a GOLD MEDAL/Scholarship from Home Minister in my MBA for being the “Top Rank student “ of management institute. 3. I received numerous complements and extra pay from various students for trouble shooting their online problems. Other interests/Hobbies (optional): ? Web Surfing ? Sports ? Watching Comics, News channels ? Miniature Collection ? Exploring hidden facts ? Solving riddles and puzzles
4.80+
391+ Reviews
552+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The heat capacity of anhydrous potassium hexacyanoferrate (II) varies with temperature as follows: TIK Cp,m/J K-I mol-I) TIK Cp,m/JK-I mol-I) 10 2.09 100 179.6 20 14.43 110 192.8 30 36.44 150 237.6...
-
Two formulas for the heat capacity of CO are given here: Cp [cal/(mol C)] = 6.890 + 0.001436T (C) Cp (Btu/(lb-moleF)] = 6.864 + 0.0007978T (F) Starting with the first formula, derive the second....
-
A small pharmaceutical firm plans to manufacture a new drug and has hired you as a consultant to design a condenser to remove the drug from a gasvapor mixture. The mixture, which contains 20 mole% of...
-
Wical Rental Management Services manages four apartment buildings, each with a different owner. Wicals CEO has observed that the apartment buildings with more expensive rental rates tend to require...
-
Spice Inc.'s unit selling price is $60, the unit variable costs are $35, fixed costs are $125,000 and current sales are 10,000 units. How much will operating income change is sales increase by 8,000...
-
Simplify the given expressions. For those with exponents, express each result with only positive exponents. For the radicals, rationalize the denominator where applicable. 27a4b 3
-
What is VTL?
-
Table 11.3.2 shows the on-time performance of nine airlines, both for one month (May 2010) and for the preceding four (January to April 2010). These numbers represent percentages of flights that...
-
Jacob is a member of WCC (an LLC taxed as a partnership). Jacob was allocated $60,000 of business income from WCC for the year. Jacobs marginal income tax rate is 37 percent. The business allocation...
-
Sean Browne owns and manages a computer repair service, which had the following trial balance on December 31, 2016 (the end of its fiscal year). Summarized transactions for January 2017 were as...
-
Calculate the average CH bond enthalpy in methane using the data tables. Calculate the percent error in equating the average CH bond energy in Table 4.3 with the bond enthalpy. Table 4.3 Selected...
-
The following cyclic ether can be prepared via an intramolecular Williamson ether synthesis. Show what reagents you would use to make this ether.
-
A box contains 5 red and 5 blue marbles. Two marbles are withdrawn randomly. If they are the same color, then you win $1.10; if they are different colors, then you win $1.00. (That is, you lose...
-
Solve the Differential equation. xydx+dy=0
-
What is Hue and saturation?
-
Explain traversing on the following parcel.Provide one numerical example.
-
Explore the role of post-occupancy evaluation in commercial and industrial architecture. How do architects use feedback from building users to improve future designs?
-
Analyze the impact of sustainable construction on biodiversity and ecosystem services. How can construction practices be adapted to minimize impacts on local ecosystems and enhance biodiversity?
-
Menaga Company reports the following information pertaining to its operating activities: During the year, the company purchased $175,000 of direct materials and incurred $45,000 of direct labor...
-
QUESTION 2 The CEO of Farisha Hijab Sdn Bhd insisted on further investigation to be carried out that he also required Mr Muaz to conduct the analysis of variance for the material and labour of the...
-
In which of the following systems is the energy level separation the largest? (a) A 14Nnucleus in (for protons) a 600 MHz NMR spectrometer, (b) An electron in a radical in a field of 0.300 T
-
Calculate the magnetic field needed to satisfy the resonance condition for unshielded protons in a 150.0 MHz radiofrequency field.
-
Use Table 15.2 to predict the magnetic fields at which (a) 14N, (b) 19F, and (c) 31p comes into resonance at (i) 300 MHz, (ii) 750 MHz.
-
Flexible manufacturing places new demands on the management accounting information system and how performance is evaluated. In response, a company should a. institute practices that reduce switching...
-
Revenue and expense items and components of other comprehensive income can be reported in the statement of shareholders' equity using: U.S. GAAP. IFRS. Both U.S. GAAP and IFRS. Neither U.S. GAAP nor...
-
Kirk and Spock formed the Enterprise Company in 2010 as equal owners. Kirk contributed land held an investment ($50,000 basis; $100,000 FMV), and Spock contributed $100,000 cash. The land was used in...

Study smarter with the SolutionInn App


