Calculate the average CH bond enthalpy in methane using the data tables. Calculate the percent error in
Question:
Table 4.3
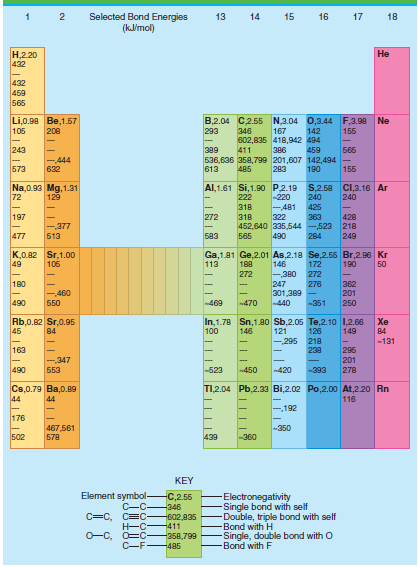
Transcribed Image Text:
Selected Bond Energies (klmol) 13 14 15 16 17 18 Н.220 432 Не 432 459 565 B,2.04 C2.55 N,3.04 0,3.44 F,3.98 Ne Li,0.90 Be,1.57 105 208 293 346 167 142 155 602,835 418,942 494 243 Б65 389 411 386 459 -444 536,636 358,799 201,607 142,494 155 573 632 613 485 283 190 Al,1.61 Si,1.90 P.2.19 S,2.58 C,3.16 Ar Na,0.93 Mg, 1.31 72 129 222 -220 240 240 -481 322 363 452,640 335,544 -523 218 318 425 197 272 318 428 -377 477 583 249 513 565 490 284 K,0.82 Sr,1.00 49 Ga,1.81 Ge,2.01 As,2.18 Se,2.55 Br,2.96 Kr 146 106 113 188 172 190 50 272 -380 272 180 247 362 201 250 276 301,389 - -440 -460 490 Б50 -469 470 -351 In,1.78 Sn,1.80 Sb,2.05 Te,2.10 1,2.66 121 -295 Rb,0.82 Sr,0.95 45 Xe 100 149 84 146 126 84 218 238 -131 163 296 201 278 -,347 553 490 -523 -420 450 -393 TI,2.04 Pb,2.33 Bi,2.02 Po,2.00o At,220 Rn Cs,0.79 Ba,0.89 44 116 44 192 176 -350 467,561 578 502 439 -360 KEY Element symbol- C-C C=C, C=C Electronegativity Single bond with self Double, triple bond with self Bond with H Single, double bond with O -Bond with F C,2.55 -346 602,835 -411 O-C, O=C- -358,799 C-F485
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 42% (7 reviews)
CH 4 gCg 4Hg H o R 4H o f H g H o f C g H o f CH ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Average bond enthalpies are generally defined for gas-phase molecules. Many substances are liquids in their standard state. By using appropriate thermo-chemical data from Appendix C, calculate...
-
Given the data in Table 4.1 (Appendix B, Data Tables) and the following information, calculate the single bond enthalpies and energies for SiF, SiCl, CF, NF, OF, HF: HF(g) SiF,(g) SiCl,(g) CF,(g)...
-
Nitrogen is a vital component of proteins and nucleic acids, and thus is necessary for life. The atmosphere is composed of roughly 80% N2, but most organisms cannot directly utilize N 2 for...
-
Jim Redman purchased a car from Bill Branch Chevrolet. However, the car had been reported stolen and Jim was unable to secure a good title. Redman sued Bill Branch Chevrolet on the breach of the...
-
Vision statements should be massively inspiring, overarching, and long term. a) Provide several examples of potential vision statements for various organizations and discuss how such vision...
-
Perform the indicated operations, expressing answers in simplest form with rationalized denominators. (43) 2
-
What is a split bill?
-
Consider each of the following independent situations: a. A computer service agreement in which a company pays $150 per month and $15 per hour of technical time. b. Fuel cost of the companys fleet of...
-
Silver Company makes a product that is very popular as a Mothers Day gift. Thus, peak sales occur in May of each year, as shown in the companys sales budget for the second quarter given below: April...
-
Prepare the 2021 statement of cash flows for Smolira Golf Corp. Some recent financial statements for Smolira Golf Corp. follow. Use this information to work this problem. SMOLIRA GOLF CORP. 2020 and...
-
Show what reagents you would use to prepare each of the following ethers via a Williamson ether synthesis, and explain your reasoning. a. b. c. OMe
-
The heat capacity of α -quartz is given by The coefficient of thermal expansion is given by β = 0.3530 à 10 4 K 1 and V m = 22.6 cm 3 mol+. Calculate ÎS m for...
-
Why do some governmental units not report small amounts of inventories of supplies in their balance sheets?
-
Define subjective brightness and brightness adaptation?
-
Write Down The Properties Of Haar Transform?
-
Explain Spatial Filtering?
-
What Is Maximum Filter And Minimum Filter?
-
Name The Categories Of Image Enhancement And Explain?
-
The Wadena Company reports the following information pertaining to the month of January: During January, the company purchased $40,000 of direct materials and incurred $90,000 of direct labor costs....
-
What is your opinion of advertising awards, such as the Cannes Lions, that are based solely on creativity? If you were a marketer looking for an agency, would you take these creative awards into...
-
What is the resonance frequency of a proton in a magnetic field of 14.1 T?
-
33S has a nuclear spin of% and a nuclear g-factor of 0.4289 calculate the energies of the nuclear spin states in a magnetic field of7.500 T.
-
Calculate the frequency separation of the nuclear spin levels of a 14N nucleus in a magnetic field of 15.4 T given that the magnetogyricrati ratio is 1.93 X 107 T-1 s-l
-
Break-Even Sales and Sales to Realize Income from Operations For the current year ending October 31, Yentling Company expects fixed costs of $537,600, a unit variable cost of $50, and a unit selling...
-
You buy a stock for $35 per share. One year later you receive a dividend of $3.50 per share and sell the stock for $30 per share. What is your total rate of return on this investment? What is your...
-
Filippucci Company used a budgeted indirect-cost rate for its manufacturing operations, the amount allocated ($200,000) is different from the actual amount incurred ($225,000). Ending balances in the...

Study smarter with the SolutionInn App


