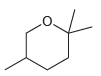
The following cyclic ether can be prepared via an intramolecular Williamson ether synthesis. Show what reagents you
Question:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (15 reviews)
Ho...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Each of the following alcohols can be prepared via reduction of a ketone or aldehyde. In each case, identify the aldehyde or ketone that would be required. a. b. c. d. .
-
Each of the following alcohols can be prepared via reduction of a ketone or aldehyde. In each case, identify the aldehyde or ketone that would be required. a. b. c. d. .
-
Each of the following alcohols can be prepared via reduction of a ketone or aldehyde. In each case, identify the aldehyde or ketone that would be required. a. b. c. d. HO. HO.
-
Which of the following is not necessary to do before you can run a Java program? a. Coding b. Compiling c. Debugging d. Saving
-
Salmon Inc. has debt with both a face and a market value of $3,000. This debt has a coupon rate of 7% and pays interest annually. The expected earnings before interest and taxes is $1,200, the tax...
-
Express each expression in simplest form with only positive exponents. 3(25) 3/2
-
How is the double entry completed when the VTL is maintained?
-
A solution of diphenyl (MW = 154.2) in benzene is formed by mixing 56.0 g diphenyl with 550.0 mL of benzene. Estimate the effective vapor pressure of the solution at 30C and the melting and boiling...
-
Determine the future value of the following two investments. Assume equal payments at the end of each period for each investment option. Note: Do not use a negative sign with your answers.
-
Pretzel Company acquired the assets (except for cash) and assumed the liabilities of Salt Company on January 2, 2020. As compensation, Pretzel Company gave 30,000 shares of its common stock, 15,000...
-
The heat capacity of α -quartz is given by The coefficient of thermal expansion is given by β = 0.3530 à 10 4 K 1 and V m = 22.6 cm 3 mol+. Calculate ÎS m for...
-
Does the enthalpy of formation of H 2 O(l) change if the absolute enthalpies of H 2 (g) and O 2 (g) are set equal to 100. kJ mol 1 rather than to zero? Answer the same question for CO 2 (g). Will H o...
-
Suppose that lithium-ion batteries are produced by three firms, one each in Countries A, B, and C. The firms are called Firm A, Firm, B, and Firm C, respectively. Each firm produces with a marginal...
-
Civil What are the challenges of using intermediate structures in the analysis of Pauli structures?
-
Civil What are the advantages of intermediate structures in the analysis of Laguerre structures?
-
Civil What are the disadvantages of using intermediate structures in the analysis of geothermal energy environmental product promotion?
-
Civil How can intermediate structures be used in the analysis of geothermal energy environmental product pulmonary toxins?
-
Civil What challenges arise when using intermediate structures in the analysis of structural robustness?
-
Sonic Marine produces sonar fishing equipment. The companys F5-300 Sonar units are produced in a single manufacturing department. All direct material used in the production of these units is added at...
-
Evenflow Power Co. is considering a new project that is a little riskier than the current operations of the company. Thus, management has decided to add an additional 1.5% to the company's overall...
-
Amine bases in nucleic acids can react with alkylating agents in typical SN2 reactions. Look at the following electrostatic potential maps, and tell which is the better nucleophile, guanine or...
-
Human brain natriuretic peptide (BNP) is a small peptide of 32 amino acids used in the treatment of congestive heat failure. How many nitrogen bases are present in the DNA that codes for BNP?
-
Human and horse insulin both have two polypeptide chains, with one chain containing 21 amino acids and the other containing 30 amino acids. They differ in primary structure at two places. At position...
-
Pedro lives in Puerto Rico and had a net taxable income of $35,000 for the year 20X1. Your gross income totals $60,000. What is Pedro's regular income tax for 20X1? a.$4,620 b.$4,900 c.$2,318 d.$2,520
-
The change in cash is equal to the change in liabilities less the change in equity plus the change in noncash assets. O True False
-
Tom holds a 5-yr 10%-coupon bond, while his friend Jackson holds a 6- year 8%-coupon bond. They are both concerned about interest rate risk but they do not fully understand how it affects them. Can...

Study smarter with the SolutionInn App


