Consider the following table of diatomic molecules and associated rotational constants: a. Calculate the rotational temperature for
Question:
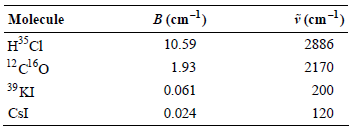
a. Calculate the rotational temperature for each molecule.
b. Assuming that these species remain gaseous at 100 K, for which species is the equipartition theorem prediction for the rotational contribution to the internal energy appropriate?
c. Calculate the vibrational temperature for each molecule.
d. If these species were to remain gaseous at 1000 K, for which species is the equipartition theorem prediction for the vibrational contribution to the internal energy appropriate?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





