Question: The lowest four energy levels for atomic vanadium (V) have the following energies and degeneracies: What is the contribution to the average energy from electronic
The lowest four energy levels for atomic vanadium (V) have the following energies and degeneracies:
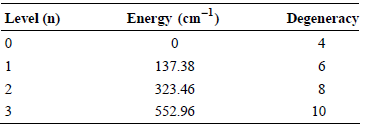
What is the contribution to the average energy from electronic degrees of freedom for V when T = 298 K?
Level (n) Energy (cm-1) Degeneracy 4 137.38 6 323.46 552.96 3 10
Step by Step Solution
3.42 Rating (183 Votes )
There are 3 Steps involved in it
Converting to J U N 143 cm 1 hc nN A 143 cm 1 662610 34 j s 30010 10 cm s 1 n ... View full answer

Get step-by-step solutions from verified subject matter experts


