The three lowest energy levels for atomic carbon (C) have the following energies and degeneracies: Determine the
Question:
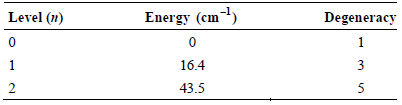
Determine the electronic contribution to CV for atomic C at 100. K.
Transcribed Image Text:
Level (n) Energy (cm) Degeneracy 1 1 16.4 3 43.5 3. 2.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (14 reviews)
Beginning the expression for internal energy derived in ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The lowest four energy levels for atomic vanadium (V) have the following energies and degeneracies: What is the contribution to the average energy from electronic degrees of freedom for V when T =...
-
Calculate the three lowest energy levels, together with their degeneracies, for the following systems (assume equal mass distinguishable particles): a. Three non-interacting spin particles in a box...
-
The molar heat capacities for carbon dioxide at 298.0 K are Cv = 28.95 J K-1 mol-1 Cp = 37.27 J K-1mol-1 The molar entropy of carbon dioxide gas at 298.0 K and 1.000 atm is 213.64 JK-1mol-1. a....
-
Mrs Anh Thuy is a 43 year old lady admitted following an incidence of blurred vision, numbness down the right side and a sharp pain in her head. A neighbour found her on the ground unable to move or...
-
(a) state the domain of the function, (b) identify all intercepts, (c) find any vertical or slant asymptotes, and (d) plot additional solution points as needed to sketch the graph of the rational...
-
According to Figure 1, a Fauna H rock would most likely form under which of the following pressure and temperature conditions? Pressure Temperature A. 4 psi 200 C B. 8 psi 500 C C. 12 psi 350 C D....
-
A study of the effect of a products warning label to determine whether consumers will still buy the product
-
On December 23, 2012, Big Sky Sports Manufacturing sells a truckload of sporting goods to the Sports R Us store in Amarillo, Texas. The terms of the sale are FOB destination. The truck runs into bad...
-
Required information PA11-1 (Algo) Analyzing Accounting Equation Effects, Recording Journal Entries, and Preparing a Partial Balance Sheet Involving Stock Issuance and Purchase Transactions (LO 11-2)...
-
1. Write a value returning function that receives three integers and returns the largest of the three. Assume the integers are not equal to one another. 2. Write a value returning function that...
-
For an ensemble consisting of a mole of particles having two energy levels separated by 1000. cm 1 , at what temperature will the internal energy equal 3.00 kJ?
-
Carbon dioxide has attracted much recent interest as a greenhouse gas. Determine the vibrational contribution to C V for CO 2 , where 1 = 2349 cm -1 , 2 = 667 cm -1 (doubly degenerate), and 3 =...
-
A help desk devoted to student software problems also receives phone calls. The number of persons that can be served in person, within one hour, is the response y. The predictor variable, x, is the...
-
Which topics do you see as being most relevant to your current job or the job you will seek to obtain once you have earned your degree? How so ? In which ways has this course Commercial Law changed...
-
Directions Answer the following reflective questions: There do exist examples of business organizations following principles of behavior that are not entirely self-serving, but rather, are pursuing...
-
10 Count scallops cost $12.97 per pound. How much do they cost for each? A Wagyu Beef New York Strip costs $14 per pound and weighs 15 pounds. The useable yield is 12.5 pounds. How many 12 ounce...
-
How do coordinating agencies differ in a crisis, disaster, and an emergency ?Explain
-
How do we manage and respond to customer feedback and reviews to maintain a positive brand reputation? Explain with the help of examples.
-
In Exercises 2934, evaluate c F dr. F(x, y, z) = xi + yj + zk C: r(t) = 2 sin ti + 2 cos tj + tk, 0tn
-
The unadjusted trial balance of Secretarial Services is as follows: SECRETARIAL SERVICES Unadjusted Trial Balance as at 31 December 2017 Account Debit Credit Cash at bank Office supplies Prepaid...
-
Compound A is an alkene that was treated with ozone (followed by DMS) to yield only 4-heptanone. Identify the major product that is expected when compound A is treated with MCPBA followed by aqueous...
-
Identify the reagents necessary to achieve each of the following transformations: Br Br - " Br Br "Br
-
Determine the configuration for every chirality center in each of the following compounds. a. b. c. - - - - - - CH- - CH- II
-
En prenant un exemple de votre choix, montrer comment on value un swap de taux de change.
-
How much would you need to invest today in order to receive: a. $10,000 in 5 years at 11%? b. $11,000 in 12 years at 8%? c. $12,000 each year for 10 years at 8%? d. $12,000 at the beginning of each...
-
A company that manufactures pulse Doppler insertion flow meters uses the Straight Line method for book depreciation purposes. Newly acquired equipment has a first cost of $190,000 with a 3-year life...

Study smarter with the SolutionInn App


