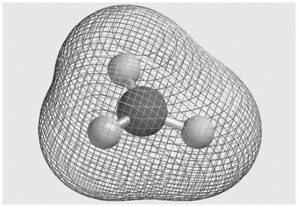
Question: Consider the molecular electrostatic potential map for the BH 3 molecule shown here. Is the hydrogen atom (shown as a white sphere) an electron acceptor
Consider the molecular electrostatic potential map for the BH3molecule shown here. Is the hydrogen atom (shown as a white sphere) an electron acceptor or an electron donor in this molecule?
Step by Step Solution
3.40 Rating (163 Votes )
There are 3 Steps involved in it
It is an electron acceptor becaus... View full answer

Get step-by-step solutions from verified subject matter experts


