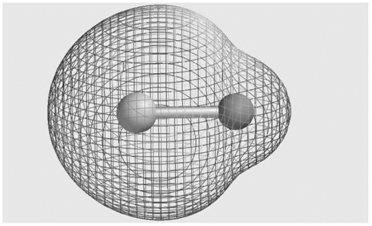
Consider the molecular electrostatic potential map for the LiH molecule shown here. Is the hydrogen atom (shown
Question:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (5 reviews)
It is an electron acceptor becaus...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the molecular electrostatic potential map for the NH 3 molecule shown here. Is the hydrogen atom (shown as a white sphere) an electron acceptor or an electron donor in this molecule?
-
Consider the molecular electrostatic potential map for the BH 3 molecule shown here. Is the hydrogen atom (shown as a white sphere) an electron acceptor or an electron donor in this molecule?
-
Consider the molecular electrostatic potential map for the BeH 2 molecule shown here. Is the hydrogen atom (shown as a white sphere) an electron acceptor or an electron donor in this molecule?
-
Workplace stress is sometimes related to time management. Describe a time when you were stressed at work or school because of time management. Explain how you would advise an employee or student to...
-
Define the inverse cosecant function by restricting the domain of the cosecant function to the intervals [/2, 0) and (0, /2], and sketch the graph of the inverse trigonometric function.
-
In Problems 21100, establish each identity. In |1 + cos0| + In|1 cos0] = 2 In|sin |
-
In fitting a least squares line to n = 20 data points, the following quantities were computed: SSxx = 60, SSyy = 225, SSxy = -75, x = 2.4, and y = 45. a. Find the least squares line. b. Graph the...
-
In the Excel file Credit Risk Data, test the hypotheses that the number of months employed is the same for applicants with low credit risk as those with high credit risk evaluations. Use a level of...
-
Exercise 12A-1 (Algo) Basic Present Value Concepts [LO12-7] Annual cash inflows that will arise from two competing investment projects are given below: The discount rate is 11%. Click here to view...
-
Determine Vo and ID for the network of Fig. 2.158. Si Si
-
The total energy of a molecule is lowered if the orbital energy of the anti-bonding MO is negative, and raised if the orbital energy of the anti-bonding MO is positive. The zero of energy is the...
-
Consider the molecular electrostatic potential map for the H 2 O molecule shown here. Is the hydrogen atom (shown as a white sphere) an electron acceptor or an electron donor in this molecule?
-
You own a portfolio that has $3,100 invested in Stock A and $4,600 invested in Stock B. If the expected returns on these stocks are 9.8 percent and 12.7 percent, respectively, what is the expected...
-
Shire Company's predetermined overhead rate is based on direct labor cost. Management estimates the company will incur $649,000 of overhead costs and $590,000 of direct labor cost for the period....
-
You plan to live 25 years after you retire. You want to withdraw $100,000 each year for 25 years. Your first withdrawal will take place the day after you retire. What is the four annuity formulas...
-
Harwood Company's quality cost report is to be based on the following data: 2021 2022 Depreciation of test equipment $94,000 $95,000 Audits of the effectiveness of the quality system $54,000 $51,000...
-
Cash contribution of 4,000 to the Accounting Society (a charity) Purchase of art object at an Accounting Society Charitable event for $1,200 (FMV $800) Donation of 3-year-old clothing (basis 800; FMV...
-
The government is issuing $100 million in 10 year debt and receives the following bids. $25 million is reserved for non-competitive tenders. At what yield will the non-competitive tenders be issued...
-
In Exercises 5366, begin by graphing the standard quadratic function, f(x) = x 2 . Then use transformations of this graph to graph the given function. 2 h(x) = (x - 1)
-
2.) Find the Laplace transform of f(t) 7e-St cos 2t +9 sinh2 2t. Use Laplace Table. %3D
-
What irreducible representations do the four H1s orbitals of CH 4 span? Are there s and p orbitals of the central C atom that may form molecular orbitals with them? Could d orbitals, even if they...
-
Molecules belonging to the point groups D 2h or C 3h cannot be chiral. Which elements of these groups rule out chirality?
-
Explain the construction and content of a character table.
-
Slow Roll Drum Co. is evaluating the extension of credit to a new group of customers. Although these customers will provide $198,000 in additional credit sales, 13 percent are likely to be...
-
Wendell's Donut Shoppe is investigating the purchase of a new $39,600 conut-making machine. The new machine would permit the company to reduce the amount of part-time help needed, at a cost savings...
-
1.Discuss the challenges faced with Valuing Stocks and Bonds. As part of this discussion, how will the selected item be implemented in an organization and its significance? 2. Discuss how Valuing...

Study smarter with the SolutionInn App


