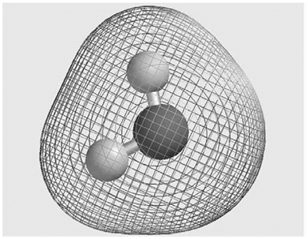
Consider the molecular electrostatic potential map for the H 2 O molecule shown here. Is the hydrogen
Question:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (14 reviews)
It is an electron donor because t...View the full answer

Answered By

Ali Khawaja
my expertise are as follows: financial accounting : - journal entries - financial statements including balance sheet, profit & loss account, cash flow statement & statement of changes in equity -consolidated statement of financial position. -ratio analysis -depreciation methods -accounting concepts -understanding and application of all international financial reporting standards (ifrs) -international accounting standards (ias) -etc business analysis : -business strategy -strategic choices -business processes -e-business -e-marketing -project management -finance -hrm financial management : -project appraisal -capital budgeting -net present value (npv) -internal rate of return (irr) -net present value(npv) -payback period -strategic position -strategic choices -information technology -project management -finance -human resource management auditing: -internal audit -external audit -substantive procedures -analytic procedures -designing and assessment of internal controls -developing the flow charts & data flow diagrams -audit reports -engagement letter -materiality economics: -micro -macro -game theory -econometric -mathematical application in economics -empirical macroeconomics -international trade -international political economy -monetary theory and policy -public economics ,business law, and all regarding commerce
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the molecular electrostatic potential map for the NH 3 molecule shown here. Is the hydrogen atom (shown as a white sphere) an electron acceptor or an electron donor in this molecule?
-
Consider the molecular electrostatic potential map for the BH 3 molecule shown here. Is the hydrogen atom (shown as a white sphere) an electron acceptor or an electron donor in this molecule?
-
Consider the molecular electrostatic potential map for the BeH 2 molecule shown here. Is the hydrogen atom (shown as a white sphere) an electron acceptor or an electron donor in this molecule?
-
Audrey has recently purchased a home with a $325,000 mortgage. She opted for monthly payments, a term of 5 years at a rate of 3.2%, and an amortization period of 25 years. Had Audrey chosen, instead,...
-
Define the inverse secant function by restricting the domain of the secant function to the intervals [0, /2) and ( /2, ], and sketch the graph of the inverse trigonometric function.
-
Several research papers use a sinusoidal graph to model blood pressure. Assuming that a persons heart beats 70 times per minute, the blood pressure P of an individual after t seconds can be modeled...
-
Consider the following sample data: y 5 1 3 x 5 1 3 a. Construct a scatterplot for the data. LO9 b. It is possible to find many lines for which 1y - yn2 = 0. For this reason, the criterion 1y - yn2 =...
-
The Odessa Independent Phone Company (OIPC) is currently engaged in a rate case that will set rates for its Midland-Odessa area customer base. OIPC has total assets of $20 million. The Texas Public...
-
In December of 2021, XL Computer's internal auditors discovered that office equipment costing $800,000 was charged to expense in 2019. The asset had an expected life of 10 years with no residual...
-
If a merchandising company ends a period with a larger inventory than it owned at the beginning of the period, then: A) The cost of goods sold was larger than net purchases. B) Net income was larger...
-
Consider the molecular electrostatic potential map for the LiH molecule shown here. Is the hydrogen atom (shown as a white sphere) an electron acceptor or an electron donor in this molecule?
-
For the case of two H1s AOs, the value of the overlap integral S ab is never exactly zero even at very large separation of the H atoms. Explain this statement.
-
Greg and Tanya Ridpath have two children, ages 6 years and 5 months. Their younger child, Ray, was born with a congenital heart defect that will require several major surgeries in the next few years...
-
Carrie enjoyed observing wildlife in natural habitats. She wanted to be able to hide at a distance but observe wildlife close up in a variety of circumstances. She visited five different shops and...
-
The operating budget for a certain company shows a net income of $350,000. To achieve this, the company is targeting sales of $637,000, variable costs of $280,280, and fixed costs of $6,720. Compute...
-
What is the output? for (i=0; i
-
a) For silicon, if EG decreases by 0.078 eV, by what fraction does n; increase (assume T is constant at 300K)? b) If the temperature rises from 300K to 600K, by what additional fraction does n;...
-
LOTR (1p) Frodo and Sam need to get to Mordor to destroy the One Ring, but they don't know the exact way. They met a creature called Gollum who claims to know the best way to Mordor. Gollum is...
-
In Exercises 5364, complete the square and write the equation in standard form. Then give the center and radius of each circle and graph the equation. x + y2 + 12x - - 4 = 0
-
Fahrad Inc. sells all of its product on account. Fahrad has the following accounts receivable payment experience: Percent paid in the month of sale .........10 Percent paid in the month after the...
-
The (one-dimensional) matrices D(C 3 ) = 1 and D(C 2 ) = 1, and D(C 3 ) = 1 and D(C 2 ) = 1 both represent the group multiplication C 3 C 2 = C 6 in the group C 6 v with D(C 6 ) = +1 and 1,...
-
Show that the function xy has symmetry species B 2 in the group C 4v .
-
Show that the transition A 1 A 2 is forbidden for electric dipole transitions in a C 3v molecule.
-
help me A 35% discount on 3 smart phone amounts to $385. What is the phone's list price? Answer =$ (rounded to the nearest cent)
-
What effect is there on the income statement and balance sheet when an expense is left too long as a liability
-
Which of the following programs covers custodial care? A HMOs B Medicare Part B C PPOs D Medicare Part A E Medicaid

Study smarter with the SolutionInn App


