Consider the reaction TiO 2 (s) + 2 C(graphite) + 2 Cl 2 (g) 2 CO(g) +
Question:
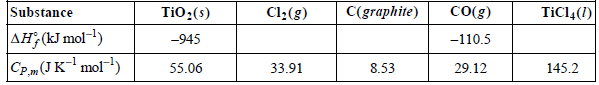
Assume that the heat capacities are independent of temperature.
a. Calculate ΔHoR at 135.8ºC, the boiling point of TiCl4.
b. Calculate ΔHof for TiCl4(l) at 25ºC.
Transcribed Image Text:
C(graphite) Co(g) TiCl4(1) TiO:(s) Cl:(g) Substance AH, (kJ mol-1) -110.5 -945 55.06 8.53 29.12 33.91 145.2 CP.(JKmol)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 87% (8 reviews)
a In this case the heat capacities are assumed to be in...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
At 1000.K, H o R = 123.77 kJ mol 1 for the reaction N 2 (g) + 3H 2 (g) 2NH 3 (g), with C P,m = 3.502R, 3.466 R, and 4.217 R for N 2 (g), H 2 (g), and NH 3 (g), respectively. Calculate H o f of NH 3...
-
At 295 k, H o R = 131.28 kJ mol -1 for the reaction C (graphite) + H 2 O(g) CO(g), with C P,m = 8.53, 33.58, 29.12, and 28.82 J K -1 mol -1 for graphite. H 2 O(g), CO(g), and H 2 (g) respectively....
-
Calculate the standard enthalpy of formation of FeS 2 (s) at 600. °C from the following data at 298.15 K. Assume that the heat capacities are independent of temperature. You are also given that...
-
What is the timestep value ? And how do I go about altering from downstream to upstream? The following code solves the advection equation 1 2 3 4 5 6 7 8 9- 10 - 11 12 - 13 - 14 - 15 - 16 - 17 18 19...
-
The diagram in Figure 1 was developed for the project launch of Kitty Condo, a new product in the luxury cat cage market. Suppose that you, as project manager, are interested in finding ways to speed...
-
State the vertical asymptotes, if any exist for the function. T f(x) = x+81
-
2. What is a forward-looking multiple? Why should one use forward-looking multiples as opposed to backward-looking multiples when valuing companies?
-
A random sample of 20 independent female college-aged dancers was obtained, and their heights (in inches) were measured. Assume the population distribution is Normal. a. What is the sample mean? Is...
-
ACCT 2123 END OF TERM ASSIGNMENT (NOTE: This is a REQUIRED assignment, NOT bonus.) Joe Nix has been the Chief Financial Officer (CFO) for Ace Manufacturing for nearly 20 years. Ace Manufacturing owns...
-
a. To simplify the analysis, assume for now that there is virtually no turnaround time between flights so the next flight can begin as soon as the current flight ends. (If an immediate next flight is...
-
Derive the equation (H/T) V = C V + V/ from basic equations and definitions.
-
Which of the following systems are open? a) A dog, b) An incandescent light bulb c) A tomato plant d) a can of tomatoes. Explain your answers.
-
Pam Corporation paid $170,000 for an 80 percent interest in Sun Corporation on December 31, 2016, when Sun's stockholders' equity consisted of $100,000 capital stock and $50,000 retained earnings. A...
-
Perhaps we need a way to differentiate ourselves from the competition? Is it possible that we are dividing the customer's time too much? Does this mean that we should instead look to attract more...
-
Complete these answers with full paragraph sentences. 1)What are the Mission, Vision, & Values of the Palo Alto Network? 2) What are the Four Functions of Management Planning, Organizing, Leading, &...
-
One highly visible trait of a successful leader is that of role model: behavior exhibited by a leader is carefully observed and often sets the tone for the entire center. As a role model, it is...
-
Design a flowchart that illustrates the key processes and decision points within the custom leadership system, along with the various inputs and outputs. At the center of the flowchart is the leader,...
-
Prepare a sample memo to those that have been selected to serve on the "Bulletin 1" committee. Remind them of their charge and outline a calendar of meetings. Lastly, include a list of resources. ...
-
Problems 103112 are based on material learned earlier in the course. The purpose of these problems is to keep the material fresh in your mind so that you are better prepared for the final exam. Write...
-
The Heese Restaurant Group manufactures the bags of frozen French fries used at its franchised restaurants. Last week, Heeses purchased and used 101,000 pounds of potatoes at a price of $ 0.70 per...
-
Calculate the speed to which a stationary 4He atom (mass 4.0026 u) would be accelerated if it absorbed each of the photons used in Exercise S.4b
-
A photon-powered spacecraft of mass 10.0 kg emits radiation of wavelength 225 nm with a power of 1.50 kW entirely in the backward direction. To what speed will it have accelerated after 10.0 y if...
-
A laser used to read CDs emits red light of wavelength 700 nm. How many photons does it emit each second if its power is? (a) 0.10 W, (b) LOW?
-
Indicate whether the following managerial policy increases the risk of a death spiral:Use of low operating leverage for productionGroup of answer choicesTrueFalse
-
It is typically inappropriate to include the costs of excess capacity in product prices; instead, it should be written off directly to an expense account.Group of answer choicesTrueFalse
-
Firms can avoid the death spiral by excluding excess capacity from their activity bases. Group of answer choicesTrueFalse

Study smarter with the SolutionInn App


