Determine the half-cell reactions and the overall cell reaction, calculate the cell potential, and determine the equilibrium
Question:
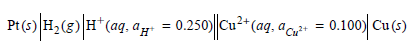
Is the cell reaction spontaneous as written?
Transcribed Image Text:
PrO|#,(e)|#"(aq, ayr = 0.250||0a*(aq. aar• = 0.100| Cu() - 0.100) Cu() = 0.250) Cu²* (ag, a c: = 0.100) Cu (s) Pt(:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (6 reviews)
We first calculate the E o for the reaction Cu 2 aq 2e Cus from the ...View the full answer

Answered By

Akshay Agarwal
I am a Post-Graduate with a specialization in Finance. I have been working in the Consulting industry for the past 8 years with a focus on the Corporate and Investment Banking domain. Additionally, I have been involved in supporting student across the globe in their academic assignments and always strive to provide high quality support in a timely manner. My notable achievements in the academic field includes serving more than 10,000 clients across geographies on various courses including Accountancy, Finance, Management among other subjects. I always strive to serve my clients in the best possible way ensuring high quality and well explained solutions, which ensures high grades for the students along-with ensuring complete understanding of the subject matter for them. Further, I also believe in making myself available to the students for any follow-ups and ensures complete support and cooperation throughout the project cycle. My passion in the academic field coupled with my educational qualification and industry experience has proved to be instrumental in my success and has helped me stand out of the rest. Looking forward to have a fruitful experience and a cordial working relationship.
5.00+
179+ Reviews
294+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
At 298 K a cell reaction has a standard cell potential of +0.17 V. The equilibrium constant for the reaction is 5.5 105. What is the value of n for the reaction?
-
The overall reaction and standard cell potential at 25oC for the rechargeable nickelcadmium alkaline battery is Cd(s) + NiO2(s) + 2H2O(l) Ni(OH)2(s) + Cd(OH)2(s) o = 1.10 V For every mole of Cd...
-
Determine the equilibrium constant for the reaction CH4 + 2O2 CO2 + 2H2O when the reaction occurs at 100 kPa and 2000 K. The natural logarithms of the equilibrium constant for the reaction C + 2H2...
-
Harvold Company's quality cost report is to be based on the following data: Test and inspection of incoming materials. $71,000 Supplies used in testing and inspection . Re-entering data because of...
-
Wheatmore Company manufactures cold cereal products. Assume that the inventory in process on January 1 for the Packing Department included 1,290 pounds of cereal in the packing machine hopper, enough...
-
Find an equation in rectangular coordinates for the surface represented by the cylindrical equation, and sketch its graph. z = r 2 sin 2 + 3r cos
-
Analyzing Employee Benefits. Talk with people employed in various types of organizations. Prepare a list of the most common types of employee benefits received by workers. Using Personal Financial...
-
Sea Duds is retail clothing store specializing in resort clothing. The company purchases finished goods apparel and accessories, and its other payments are typical operating costs such as rent,...
-
Which type of risk would be classified as diversifiable risk?
-
1. Based on what you read in this chapter, what would you have suggested Lisa and her team do first with respect to training, particularly in terms of the companys strategy? Why? 2. Have Lisa and the...
-
Table 2.10 presents data on mean SAT reasoning test scores classified by income for three kinds of tests: critical reading, mathematics, and writing. In Example 2.2, we presented Figure 2.7, which...
-
What is the conditional expectation function or the population regression function?
-
According to Nielsen Inc., the top 20 rated TV shows for 2018, based on the share of the total available audience, are listed here. A researcher believes that viewing habits of viewers who live in...
-
A large-sized chemical company is considering investing in a project that costs `5,00,000. The estimated salvage value is zero; tax rate is 35 per cent. The company uses straight line method of...
-
From the following budgeted and actual figures, calculate and present the variances in respect of profit on sales and cost of sales. Budget: Sales, 2,000 units @ 15 each Cost of sales @ 12 each...
-
(a) From the following data of a manufacturing unit, find out (i) sales to break-even and (ii) sales to earn a profit of 8,000. (b) The following information is available for companies A and B. (i)...
-
Wowem Corporation manufactures a wide range of clothing apparel. It is a decentralized organization in which different divisions have responsibility for the manufacture and distribution of major...
-
(a) Use a molecular orbital program or input and output from software supplied by your instructor to construct a molecular orbital energy-level diagram to correlate the MO (from the output) and AO...
-
In Problems 4374, find the real solutions of each equation. x 4 - 10x 2 + 25 = 0
-
With your classmates, form small teams of skunkworks. Your task is to identify an innovation that you think would benefit your school, college, or university, and to outline an action plan for...
-
The hyperfine coupling constants observed in the radical anions (12), (13), and (14) are shown (in millitesla, mT). Use the value for the benzene radical anion to map the probability of finding the...
-
The chemical shift of the CH 3 protons in acetaldehyde (ethanal) is = 2.20 and that of the CHO proton is 9.80. What is the difference in local magnetic field between the two regions of the molecule...
-
The first generally available NMR spectrometers operated at a frequency of 60 MHz; today it is not uncommon to use a spectrometer that operates at 800 MHz. What are the relative population...
-
Comfort Golf Products is considering whether to upgrade its equipment Managers are considering two options. Equipment manufactured by Stenback Inc. costs $1,000,000 and will last five years and have...
-
Weaver Corporation had the following stock issued and outstanding at January 1, Year 1: 71,000 shares of $10 par common stock. 8,500 shares of $60 par, 6 percent, noncumulative preferred stock. On...
-
Read the following case and then answer questions On 1 January 2016 a company purchased a machine at a cost of $3,000. Its useful life is estimated to be 10 years and then it has a residual value of...

Study smarter with the SolutionInn App


