DielsAlder cycloaddition of 1, 3-butadiene with acrylonitrile requires that the diene be in a cis (or cis-like)
Question:
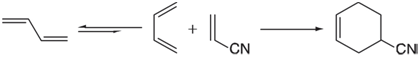
In fact, the diene exists primarily in a trans conformation, the cis conformer being approximately 9 kJ/mol less stable and separated from the trans conformer by a low-energy barrier. At room temperature, only about 5% of butadiene molecules will be in a cis conformation. Clearly, rotation into a cis conformation is required before reaction can proceed. Conduct a search for a substituted 1,3-butadiene that actually prefers to exist in a cis (or cis-like) conformation as opposed to a trans conformation. The only restriction you need to be aware of is that the diene needs to be electron rich in order to be reactive. Restrict your search to alkyl and alkoxy substituents as well as halogen. Use the HF/3-21G model. Report your successes and provide rationales.
Step by Step Answer:






