Olefins assume planar (or nearly planar) geometries wherever possible. This ensures maximum overlap between p orbitals and
Question:

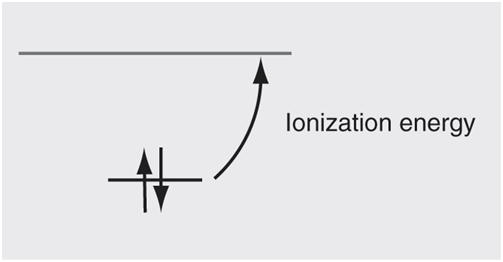
Another measure of π-bond strength, at least π-bond strength relative to a standard, is the energy required to remove an electron from the π orbital, or the ionization energy:
Non-planar olefins might be expected to result from incorporation of a trans double bond into a small ring. Small-ring cycloalkenes prefer cis double bonds, and the smallest trans cycloalkene to actually have been isolated is cyclooctene. It is known experimentally to be approximately 39 kJ/mol less stable than cis-cycclooctene. Is this a measure of reduction in π bond strength? Optimize the geometries of both cis- and trans-cyclooctene using the HF/3-21G model. (You should first examine the possible conformers available to each of the molecules.) Finally, calculate and display the HOMO for each molecule. Is the double bond in trans-cyclooctene significantly distorted from its ideal planar geometry? If so, would you characterize the distortion as puckering of the double-bond carbons or as twisting around the bond, or both? Does the HOMO in trans-cyclooctene show evidence of distortion? Elaborate. Is the energy of the HOMO in trans-cyclooctene significantly higher (less negative) than that in cis-cyclooctene? How does the energy difference compare to the experimentally measured difference in ionization potentials between the two isomers (0.29 eV)? How does the difference in HOMO energies (ionization potentials) relate to the calculated (measured) difference in isomer energies?
Step by Step Answer:






