Discuss the origin of the series of lines in the emission spectra of hydrogen. What region of
Question:
Discuss the origin of the series of lines in the emission spectra of hydrogen. What region of the electromagnetic spectrum is associated with each of the series shown in Fig. 9C.1?
Data in Fig. 9C.1
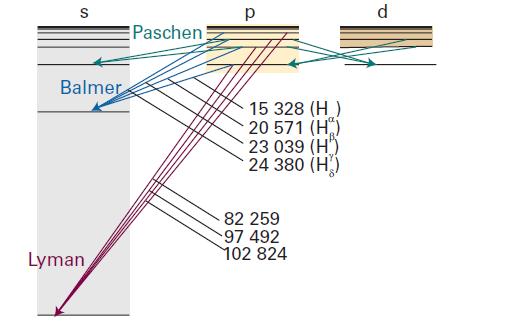
Transcribed Image Text:
S Balmer Lyman Paschen р 15 328 (H) 20 571 (H) 23 039 (H) 24 380 (H) 82 259 97 492 102 824 d
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (22 reviews)
The series of lines in the emission spectra of hydrogen are known as the hydrogen spectra They arise ...View the full answer

Answered By

BillClinton Muguai
I have been a tutor for the past 5 years. I have experience working with students in a variety of subject areas, including computer science, math, science, English, and history. I have also worked with students of all ages, from elementary school to college. In addition to my tutoring experience, I have a degree in education from a top university. This has given me a strong foundation in child development and learning theories, which I use to inform my tutoring practices.
I am patient and adaptable, and I work to create a positive and supportive learning environment for my students. I believe that all students have the ability to succeed, and it is my job to help them find and develop their strengths. I am confident in my ability to tutor students and help them achieve their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Physical Chemistry Thermodynamics And Kinetics
ISBN: 9781464124518
10th Edition
Authors: Peter Atkins, Julio De Paula
Question Posted:
Students also viewed these Sciences questions
-
What p-value statement is associated with each of the following outcomes of a hypothesis test? a. Not significant. b. Significant. c. Highly significant. d. Very highly significant.
-
The microwave region of the electromagnetic spectrum is considered to have wavelengths ranging from 5 mm to 50 cm. Calculate the range of energy of microwave photons.
-
Discuss the origin of the nuclear Overhauser effect and how it can be used to measure distances between protons in a biopolymer.
-
Recall the heat equation which we solved numerically T= = DTxx There we implemented an explicit numerical scheme (FTCS) which led to a conditionally stable solution - meaning that for certain time...
-
Jackson Corporation employs 45 production workers and pays them all the same salary. Jackson employs 10 administrative staff personnel and pays them all the same salary. The following annual...
-
Using simple moving averages and the following time series data, respond to each of the items. a. Graph the time series data. What do you observe? b. Compute all possible forecasts using a...
-
Prepare financial statements for not-for-profit colleges and universities. AppendixLO1
-
Suppose we are interested in bidding on a piece of land and we know one other bidder is interested. The seller announced that the highest bid in excess of $10,000 will be accepted. Assume that the...
-
3 24 Question 39 (1 point) ) Listen 6 27 > Under the Uniform Commercial Code, how may an acceptance be made? 9 30 1) Only in person 2 33 2) Only in writing 3) Only by traditional mail 5 36 4) Only by...
-
A graduated from the University in early 2021 at the age of 30. He immediately applied for a number of jobs and accepted a position as a financial planner in the Ottawa office of Otterbrook...
-
An electron in the ground-state He + ion undergoes a transition to a state described by the wavefunction R 4,1 (r)Y 1,1 (,). (a) Describe the transition using term symbols. (b) Compute the...
-
Identify the shortest and longest wavelength lines in the Lyman series.
-
According to Kolari, Liu, and Huang (KLH) (2021), zeta risk (denoted by Z i ,a ) in the ZCAPM can be positive or negative in sign. Illustrate this two sided zeta risk in a diagram with the ith...
-
In the following vignette write out three examples of a MI intervention in response to what the client has shared. Mark is a 19-year-old, male who lives with his parents and younger brother. His...
-
Photon Technologies, Inc., a manufacturer of batteries for mobile phones, signed a contract with a large electronics manufacturer to produce three models of lithiumion battery packs for a new line of...
-
Most human behaviour: can be easily explained has multiple causes stems from unconscious desires depends on social influence
-
In what ways are the information processing theories of cognitive development discussed in PSY393 different from the embodied cognition perspective? In what ways are they similar? In your opinion,...
-
Problem 15-6B Accounting for share investments LO4 CHECK FIGURE: 2. Carrying value per share, $19.63 River Outdoor Supply Corporation (River Corp.) was organized on January 2, 2023. River Corp....
-
A concave mirror has a focal length of 10 cm. An object is located 30 cm from the surface of the mirror. a. How far from the mirror is the image of this object? b. Is the image real or virtual,...
-
-4 1 9. Let A = Find A-1, (A") and verify that (A")= (A-1)".
-
Sketch the form of the 19 F-NMR spectra of a natural sample of tetrafluoroborate ions, BF 4 , allowing for the relative abundances of 10 BF 4 and 11 BF 4 .
-
The p-dinitrobenzene radical anion can be prepared by reduction of p-dinitrobenzene. The radical anion has two equivalent N nuclei (I = 1) and four equivalent protons. Predict the form of the EPR...
-
Sketch the appearance of the 1 H-NMR spectrum of acetaldehyde (ethanal) using J = 2.90 Hz and the data in Exercise 15.9a in a spectrometer operating at (a) 250 MHz, (b) 500 MHz. Data in Exercise...
-
If the auditor believes that the financial statements prepared on the basis of the entity's income tax are not adequately titled, the auditor should : A)Issue a resignation of opinion. B)Explain the...
-
initial stock offering to the public. This REIT specializes in the acquisition and management of warehouses. Your firm, Blue Street Advisors, is an investment management company that is considering...
-
Question 3 You have been hired to run a pension fund for Mackay Inc, a small manufacturing firm. The firm currently has Gh5 million in the fund and expects to have cash inflows of $2 million a year...

Study smarter with the SolutionInn App


